How Vegetable Oils Make Us Fat
Highlights
Over-consuming vegetable oils, specifically seed oils, which contain evolutionarily unprecedented amounts of the omega-6 fat linoleic acid, can cause obesity.
There is no tribe, population, or nation that has started consuming vegetable oils and not seen obesity rates climb.
Vegetable oil breaks down into the obesogenic toxin HNE (4-Hydroxynonenal), which appears to be a cause of obesity.
Linoleic acid sensitizes fat cells to the fat-storing hormone insulin. It can divert energy toward fat storage rather than fat-loss, up to a point.
Linoleic acid activates the body’s own (endo)cannabinoid system via the CB1 receptor in a way that may interfere with hunger signals.
For many animals, seasonal increases in linoleic acid consumption are a signal to hibernate and fatten up — might this be relevant to how human obesity develops?
Introduction
What causes obesity? It's a seemingly simple question that has a thousand different complex answers, depending on who you ask. Carbs, sugar, fat, calories, chemicals, sedentary lifestyles, food processing, and genetics have all been blamed.
The NHS in the U.K. states that "Obesity is generally caused by eating too much and moving too little," and the CDC in the U.S. seems to agree: “If you take in more calories than you use, you will gain weight [*].” However, these explanations say nothing about the underlying cause of storing excess fat. And recommendations to simply eat less and move more clearly aren’t working.
Obesity is a systemic problem, not a result of widespread loss of personal willpower to cut calories or a new unwillingness to exercise. If weight loss were as easy as deciding to ignore hunger signals and taking the stairs instead of the elevator, we would not have an obesity rate of 42.4% in the U.S., with the rest of the world quickly catching up. And we would not have an increasing percentage of American children who are overweight or obese, having grown from 36% to 46% of American kids in the last two years alone [*,*].
Something has changed in the last hundred years, during which time obesity has transitioned from anomaly to epidemic. Why have we spontaneously started storing so many calories as fat?
It has been repeatedly demonstrated in animal models and, more recently, in human studies that so-called "high-fat diets" lead to increased body fat [*]. And in mice, even when these fats bypass the mouth and are injected into the gut, the mice spontaneously start eating more [*].
However, it may not be the amount of dietary fat that causes obesity but rather the type of fat. And if American dietary trends are any indication, it’s not saturated fat, beef fat, or heavy cream that’s causing obesity, it’s vegetable oils [*,*]:
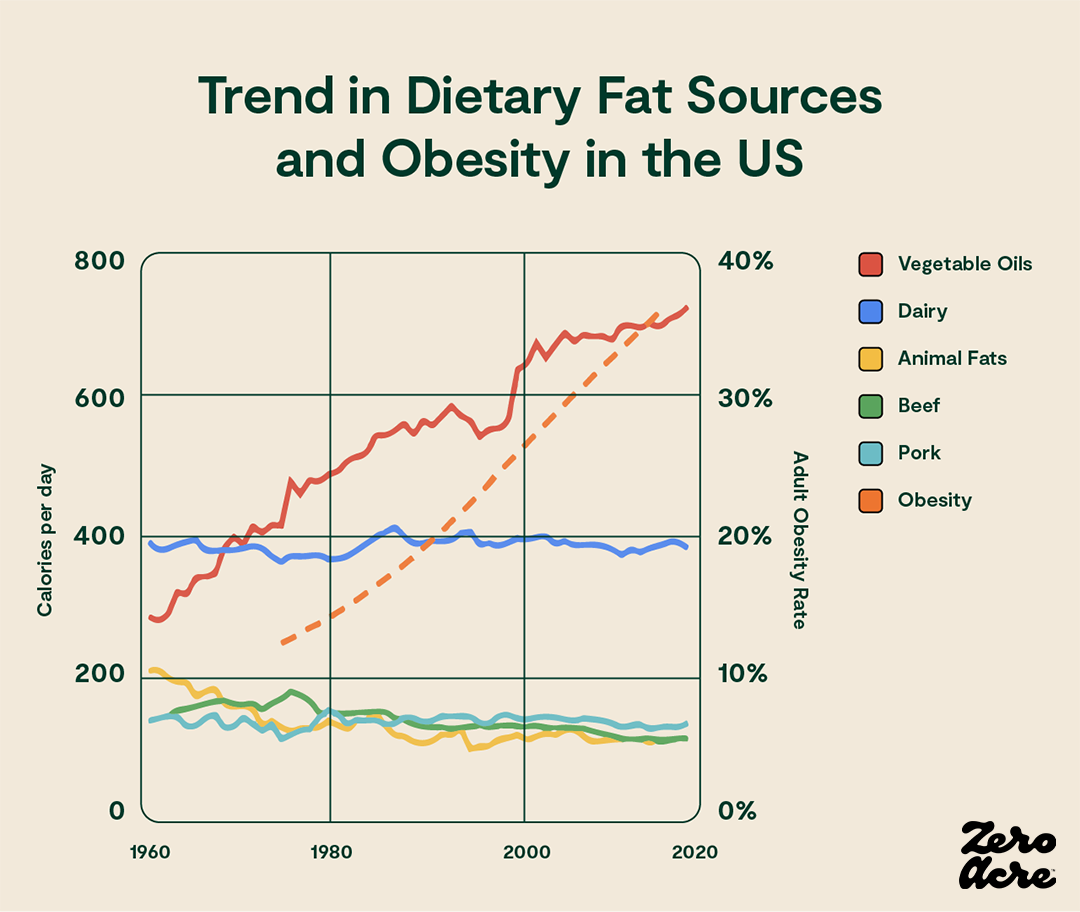
Line graph showing how much particular sources of dietary fat contributed to our daily calorie total (left Y-axis) over the years (bottom X-axis) as the adult obesity rate increased (right Y-axis).
As we'll see in the data to come, much of which has only been published in the last few years, a driving force behind increasing obesity rates may be the overconsumption of vegetable oils — soybean, sunflower, corn, and canola oil — all of which contain large amounts of linoleic acid, a type of omega-6 polyunsaturated fat.
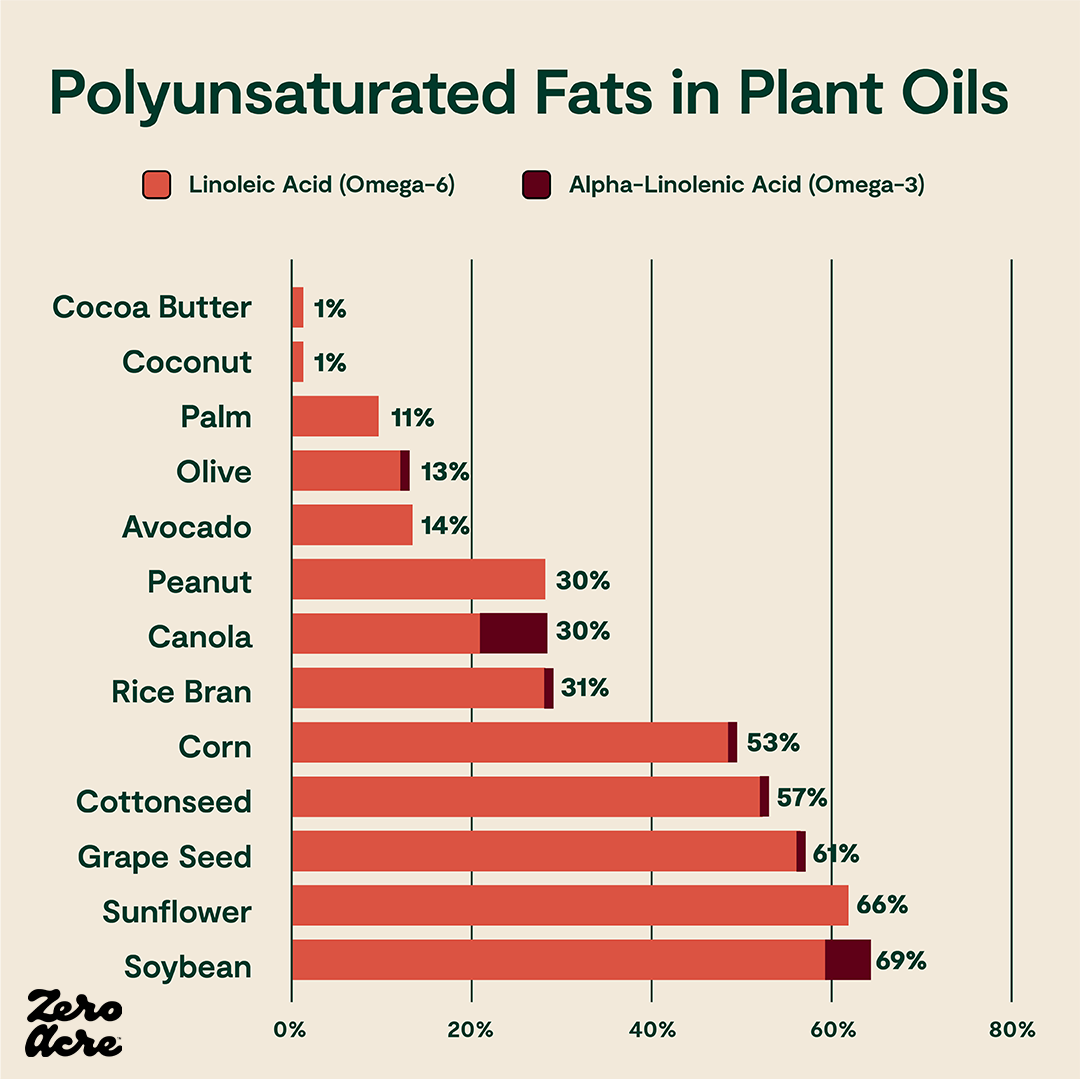
Horizontal stacked bar chart of linoleic (omega-6) and alpha-linolenic acid (omega-3) as a percentage of total calories from different sources of added fat.
Omega-6 fats, while necessary in extremely small amounts, may contribute to general inflammation and other health issues when eaten in excess [*,*]. And, as we’ll see, high levels of dietary linoleic acid can get converted to certain metabolites which activate key biological pathways that may contribute to rising obesity rates.
Vegetable Oil and The Rise in Obesity Rates
Different groups of people have eaten a myriad of diets throughout history, from predominantly carbohydrates like potatoes and honey to mostly protein and fat like meat and dairy, and obesity rates have never been as high as they are today.
There is no tribe, population, or nation that has started consuming vegetable oils and not seen obesity rates climb.
So, is it possible that rising obesity rates aren’t primarily an issue of macronutrient ratios and that there’s something else at play?
The rise in obesity is strongly associated with the rise in vegetable oil and linoleic acid consumption — arguably more so than any other dietary change — notably because there's evidence that total calories, carbohydrates, sugar, saturated fat, and red meat consumption have stayed relatively steady and in some cases have decreased while obesity rates have increased.
For example, the Kitavans of Papua New Guinea in the Pacific Islands were estimated to eat 69% of their calories as carbohydrates, primarily from sweet potatoes, of which they have an abundant caloric supply, yet they remain thin and fit [*].

Adult Kitavan man displaying the naturally lean human physique (left panel) and a young Kitavan woman. Photo by now deceased paleoanthropologist Staffan Lindeberg.
For much of the 20th century, in many East Asian countries, white rice (a refined carbohydrate) accounted for the majority of calories consumed. But even when white rice contributed 84% of daily calories, as was the case in Japan in the 1950s, most people remained thin and morbid obesity was extremely rare.
On the other end of the spectrum, and opposite side of the planet, the Inuit of Greenland traditionally ate a diet that was nearly 100% seal meat and blubber, with more than half of their calories coming from fat. Yet, just like the Kitavans and Japanese, there was no obesity epidemic. Similarly, the Massai of East Africa eat a diet composed almost entirely of blood and milk and remain fit.
You might imagine that we’re eating more today than ever, contributing to the increase in obesity rates. But in 1939, high-income Americans living in cities may have eaten even more daily calories than the average American eats today; yet an early USDA report estimated that the average man living in North Atlantic and Pacific only weighed 154 pounds [*,*,*]. The average American man today weighs 198 pounds [*].
Before Europeans brought flour and sugar to the Americas, many Native Americans subsisted on a mixed diet of corn, beans, squash, fruits, greens, nuts, meats, and maple syrup [*]. Far from overweight, Native Americans were described by early Europeans as some of the fittest people they had ever seen.
While all these healthy populations have varying diets, a major commonality is a lack of or very minimal use of vegetable oil in the diet. The consumption of dietary linoleic acid (the primary fat in many vegetable oils) was very low in pre-industrial cultures, between only 0.3-2.0% of calories [*].
Today, however, many of these cultures have begun consuming vegetable oils in massive quantities.
After industrial vegetable oils were introduced to East Asia, the Middle East, the Americas, and Pacific Island nations near Papua New Guinea, these nations saw obesity rates soar. While not every individual gets obese as a result of vegetable oil consumption, it appears that every population does.
By analogy, not everyone who smokes gets lung cancer, but every population that starts smoking sees increased lung cancer rates. Of course, different individuals have different genes. They, therefore, react to potential negative inputs — like vegetable oils and cigarettes — differently, but what we can say with confidence is that based on historical data, eating more vegetable oil is always associated with more obesity on a population level.
This population-wide association between vegetable oils and obesity may also extend to pregnant mothers and their offspring. For example, a large observational study in the U.K. found that the more linoleic acid the mother ate, the greater the child’s fat mass at both 4 and 6 years of age [*]. And a study conducted on over 500 American women in their 50s found that higher baseline levels of linoleic acid in the blood, which was representative of recent diet, were correlated with weight gain resulting in excess body weight or obesity after 10 years [*].
A study conducted in a Spanish population found a correlation between fat tissue levels of linoleic acid (which also correlated with dietary linoleic acid intake), degree of obesity, and “central adiposity,” better known as belly fat [*,*].
As you can see, there’s widespread recognition that a “dietary transition” is taking place, and one of the major elements in this transition is an increase in dietary vegetable oils [*]. In most populations, widespread weight gain (and other health issues) began occurring before the connection between vegetable oil consumption and obesity was recognized.
For example, the Tsimane are a primitive horticulturalist group living in the Bolivian Amazon jungle. They are renowned among cardiologists because they “have the lowest reported levels of coronary artery disease of any population recorded to date” [*]. Until recently, obesity was also rare among the Tsimane.
But even among the Tsimane, as they gain access to “market foods”, excess body fat is increasing. Researchers trying to discern what was driving the change in body fat found that it was only the consumption of vegetable oils [*,*,*]. The experience of the Tsimane suggests that weight gain starts quickly after the introduction of vegetable oils into a population, which is consistent with what happened in the U.S. [*]
Although observing these populations reveals clear correlations between linoleic acid and obesity, it’s essential to test the hypothesis that reducing linoleic acid reverses obesity. Well-controlled and preferably randomized clinical trials are the tool for doing that.
In one randomized trial, 41 adult women with excess body fat were given a daily high-fat breakfast for nine weeks that contained either high linoleic soybean oil or lower linoleic olive oil. Body fat loss was 80% greater in the group that consumed less linoleic acid [*].
In an even longer experiment, this time in Asian Indian males, replacing high linoleic soybean and safflower oil with lower linoleic oils for six months led to a significant decrease in body weight. The men consuming high linoleic soybean/safflower oil (60-75% linoleic acid) lost 1.7 lbs, the group consuming 21% linoleic canola oil lost 4.2 lbs, and the group consuming 11% linoleic olive oil lost 5 lbs. The less linoleic acid the men consumed, the more weight they lost [*].
The timing of widespread seed oil consumption and the link between high linoleic diets and obesity rates in the research make seed oils an excellent candidate for being a major “obesogen” — or a factor that promotes obesity by interfering with metabolism. But other than environmental changes like diet, could our genetics have turned on us and made us obese?
Genetics, Vegetable Oils, and Obesity
We know that genetics influence obesity, and it appears that vegetable oil consumption may compound the problem.
The top 10 countries with the highest rates of obesity in the world are all isolated Pacific island nations. The next 10 most obese nations are all in the Middle East, with the exception of the U.S., which is the 12th most obese country in the world [*].
Besides the U.S., every one of the world’s 25 most obese countries is either a remote island in the Pacific Ocean or a remote desert in the Middle East [*].
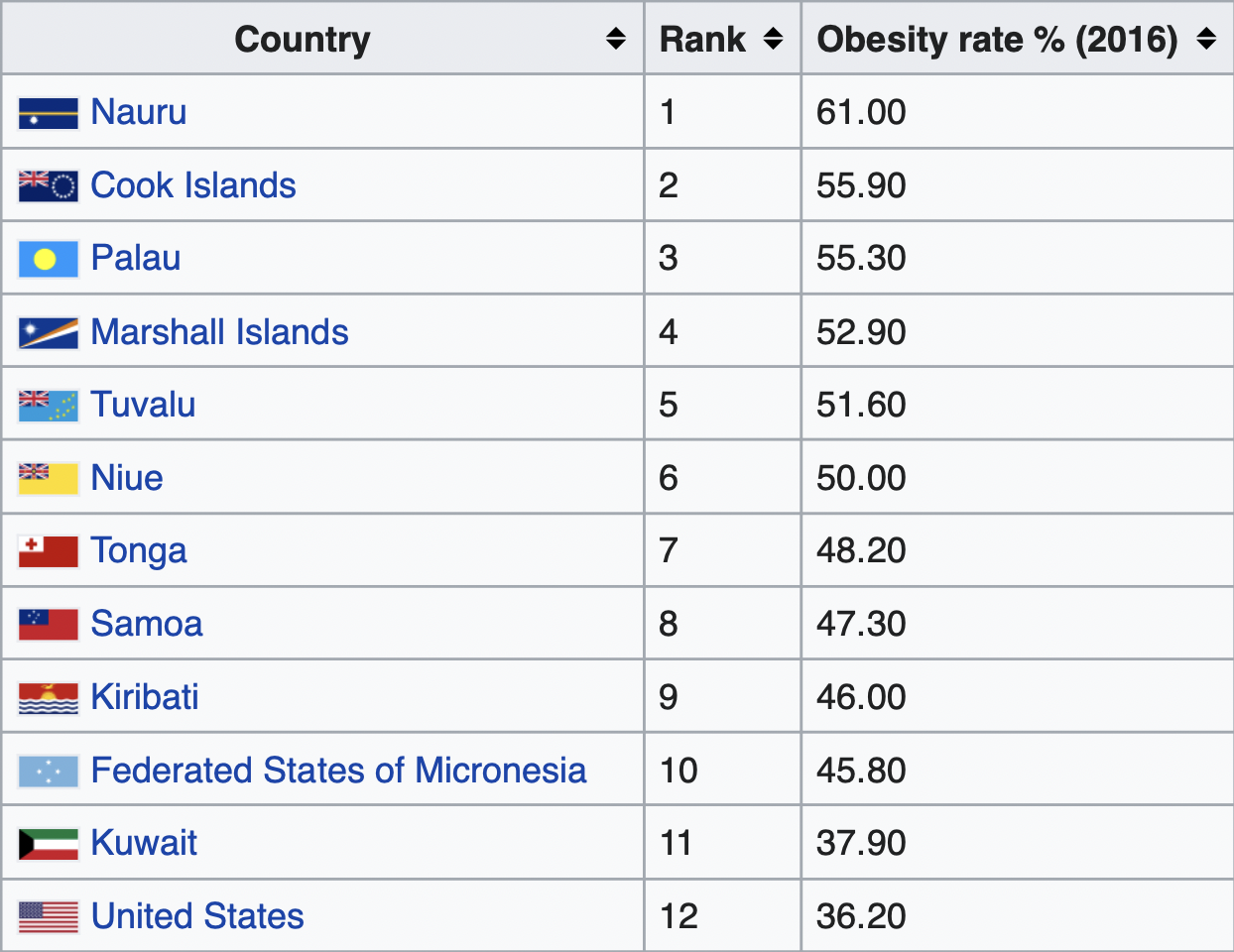
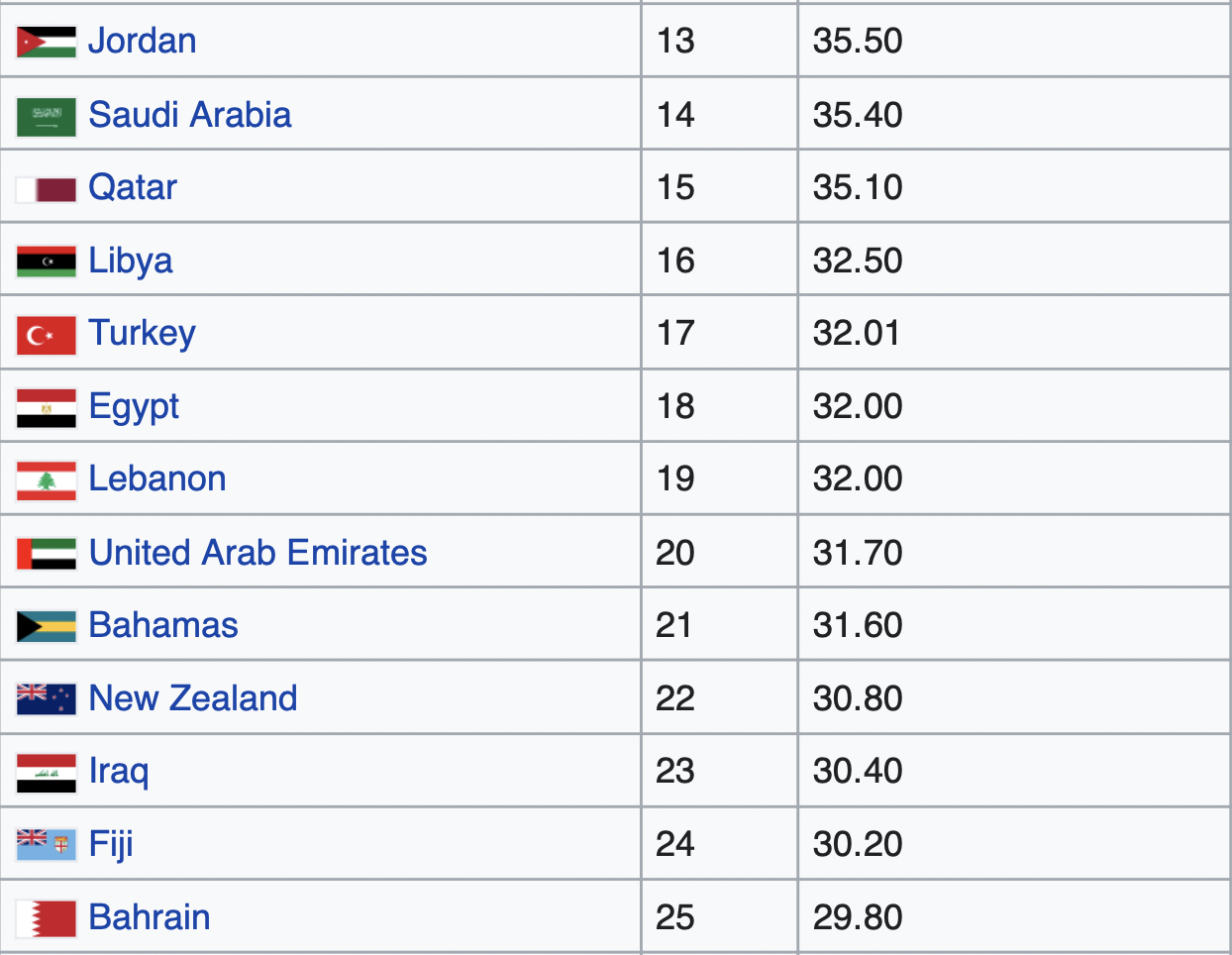
Populations across the globe ranked according to their obesity rates.
The data suggest that there's something about being a remote nation, surrounded by hundreds or thousands of miles of ocean or desert, that predisposes people to obesity. The “thrifty gene” hypothesis, whereby periods of famine select for more fat storage, is often invoked to explain why these nations’ obesity rates increased [*].
However, the originator of the hypothesis James Neel eventually admitted it wasn’t well supported, and others proposed an alternative theory called the “drifty gene [*,*].”
The “drifty gene” hypothesis described a genetic drift (i.e., random fluctuations in different versions of a gene from one generation to the next) rather than a positive selection force, leading to the accumulation of “fattening traits.” And these traits only became apparent once pushed above the threshold by environmental obesogens.
The Nauruans and Tongans in the Pacific Islands and Kuwaitis and Jordanians in the Middle East seem particularly susceptible to obesity once fast food is introduced to their environment. Whether their genetics explain this remains an open question. A VICE documentary described Kuwait, which has the highest obesity rate in the Middle East, as having an “obsession with American fast food,” which is just as processed and full of vegetable oil in the Middle East as it is in the West [*].
If there is one thing that everyone agrees on when it comes to foods that cause weight gain, it’s that so-called ultra-processed foods are especially fattening. However, researchers have not come to a consensus on what it is about processing food that makes it so obesogenic.
Interestingly, one thing that many ultra-processed foods have in common is that they’re cooked in vegetable oil, from french fries and potato chips to donuts and chicken nuggets. Researchers have shown that potatoes cooked in vegetable oil (fries and chips) are more fattening than baked and mashed potatoes, often smothered with butter and cream [*].
To prove the fattening effects of ultra-processed diets, a 2019 randomized controlled trial by NIH researcher Kevin Hall showed that an ultra-processed diet for 2 weeks caused participants to gain a pound of weight. Meanwhile, participants eating an unprocessed diet lost a pound, even when most nutrients were matched gram for gram and calorie for calorie [*].
The study’s authors point out that while they tried to match all nutrients between the two diets, there were some nutrients they couldn’t match between the two groups, including the amount of omega-6 (linoleic acid) in the diet.
The ultra-processed diet in the study had an ~11:1 omega-6 to omega-3 ratio, while the unprocessed diet had only a 5:1 ratio of omega-6 to omega-3. Since omega-3 has shown to be protective against some of the obesogenic effects of omega-6 linoleic acid, the ratio is important, and the amount of linoleic acid in the ultra-processed diet may be the cause of its fattening effects, which may also give us a hint as to what’s going on in Kuwait, the most obese country in the world outside of the Pacific Islands [*,*].**
Kuwait consumes more corn oil than any other country in the world, by nearly 400%. Kuwaitis consume 201 calories of corn oil every day, while the next most corn-oil-consuming nation, the U.S., consumes “only” 58 daily calories of corn oil [*]. Of all vegetable oils, corn oil has one of the highest amounts of omega-6 linoleic acid and lowest amounts of protective omega-3 fats, with an omega-6 to omega-3 ratio of 60:1 [*].
Corn oil is the most-consumed oil in Kuwait and also one of the first ingredients in McDonald’s french fries [*]. If Kuwaitis are following in the footsteps of American diets — based largely on ultra-processed foods, vegetable oils, and refined flours and sugars — corn oil consumption and diet-induced obesity are two areas where they’ve now surpassed the U.S.
Just like Kuwait, Pacific Island nations eat an abundance of processed foods. In Nauru, the most obese country in the world, “a reliance on imported food and [vegetable] oils” was cited as far back as 2007 as the main cause for the high level of overweight Nauruans [*]. Numerous other articles have linked Nauru’s obesity epidemic and its importation of Western fast food and processed foods [*,*,*].
Similarly, the Native American Pima tribe is experiencing an obesity crisis on a Western diet. If the Native American Pima tribe were a nation, it would be the most obese in the world, ahead of Nauru.
The Pima tribe has a 67% obesity rate and for decades now has been eating a more Western diet high in vegetable oil. As early as 1996, Pima Americans ate similar ratios of fats as other increasingly obese Americans, likely due to new dietary staples like fried potatoes and fried bread [*,*]. The historical diet of the Pima tribe had been replaced with a diet high in vegetable oil and flour, and obesity quickly followed [*]. However, Pima Mexicans with identical genetics who still live off of their traditional diet consisting of minimal vegetable oil struggle far less with obesity [*].
So, genetics may play a role in the question of what causes obesity, but only to the extent that obesogenic foods are present in the diet. Without those foods, even populations predisposed to obesity remain thin and fit.
Nations struggling with high obesity rates may not always eat more vegetable oil than other slimmer nations, but they appear genetically predisposed to store that vegetable oil (and the carbohydrates they eat along with it) as body fat. And on diets without vegetable oil, they appear to remain slim. While there are no genes that necessarily cause obesity, there are genes that influence what happens to calories once they enter the body.
Beyond our genes, other factors such as calories and exercise are often blamed for population-wide increases in obesity rates; however, at least in the U.S., calorie consumption and exercise rates have remained steady while obesity rates have climbed.
How Do Vegetable Oils Affect Energy Expenditure?
Total calories consumed may be far less important than the type of calories consumed when it comes to weight gain. In the U.S., for example, average caloric consumption has remained flat in the past couple decades while obesity rates climbed significantly.
While total caloric consumption in the U.S. increased in the late 20th century, it hasn’t changed much since the early 2000s. In fact, Americans actually consumed more calories between 2002 and 2006 than between 2014 and 2018 [*,*]. However, during that same time, obesity rates increased from 30.5% of American adults in 2002 to 42.4% in 2018, a relative increase of nearly 40% [*,*].
While total caloric intake has remained somewhat flat for the last couple of decades in the U.S., calories from vegetable oils have increased by about 30%. And calories from all other food sources have decreased since the mid-1990s, while obesity rates have soared. Calories from vegetable oils may be especially fattening.
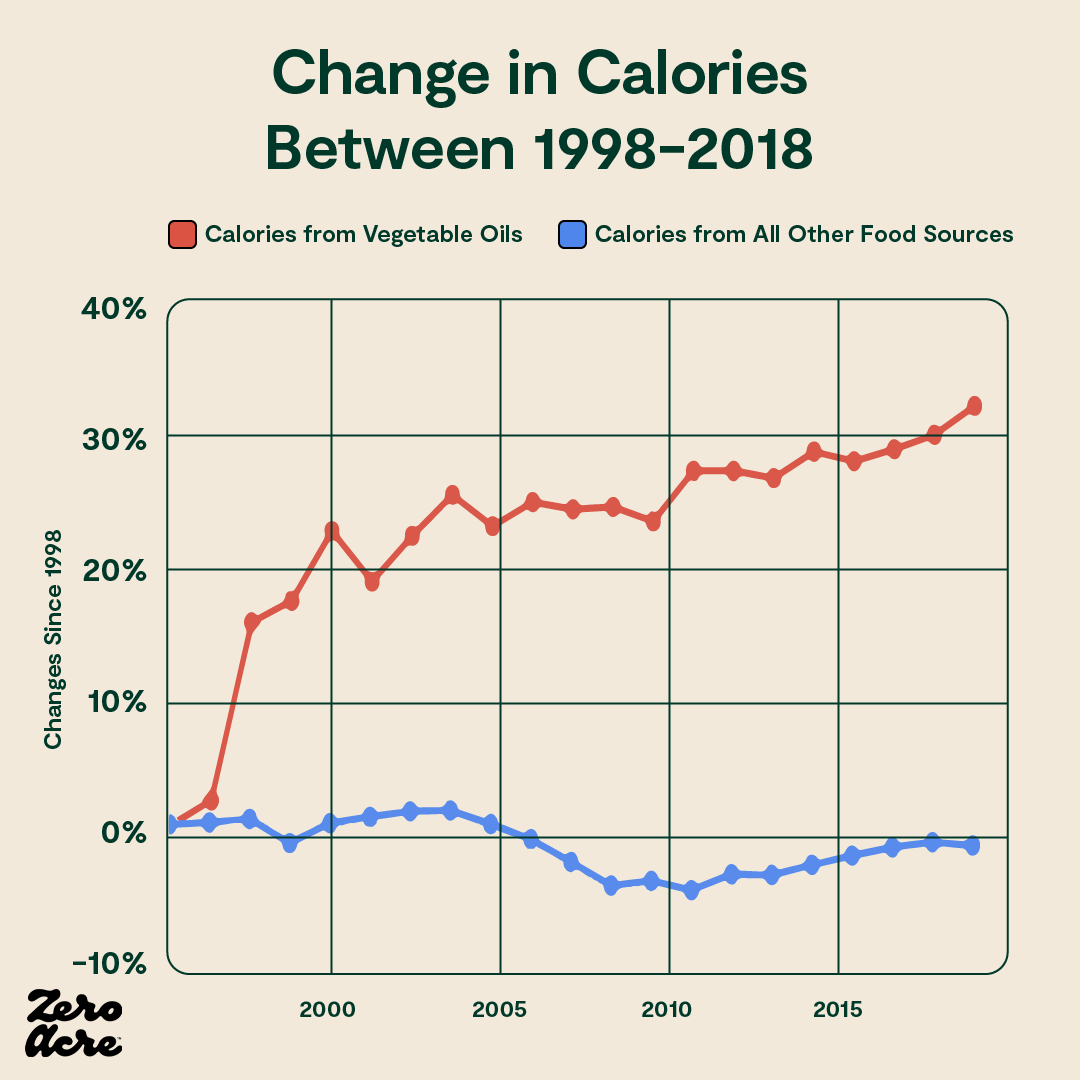
Similarly, in Japan, calories have been going down while weight gain rises. In fact, daily calorie intake was the same in 1975 as it was in 2013 in Japan, while obesity rates nearly quadrupled [*,*]. During that same time, Japan’s vegetable oil intake doubled, and since 1950 has increased 13-fold.
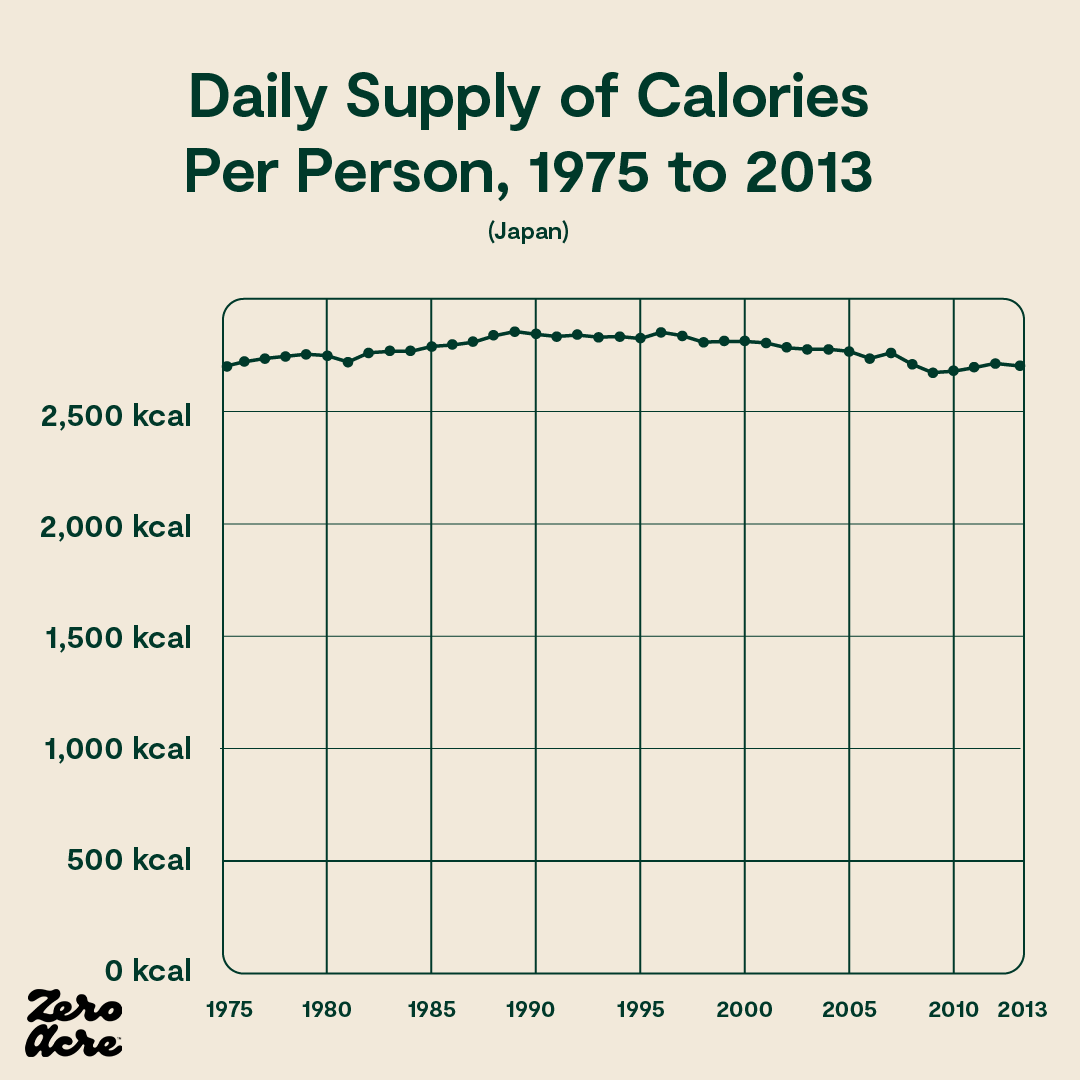

Source: OurWorldinData.org
But what about exercise? Most people think of exercise as the primary driver of calories burned, but to add to the complexity, Americans have steadily been exercising more over the last 20 years [*]:

While exercise plays a role in how many daily calories we burn, it is only a minor one.
For most people with sedentary lifestyles, only 2-3% of all calories burned in a day are from exercise. Even in manual workers, who essentially spend all day exercising, only about 30% of calories burned are from active movement.
The other 70-98% of our energy (calorie) expenditure is simply a result of being a warm-blooded animal with a big brain. We burn calories to keep our bodies warm, circulate blood, breathe, think, open the fridge, digest food, and go to the bathroom. The rate at which we burn calories just by living and being human is called our “metabolic rate.”
At this point, you might ask, if our calorie intake has stayed the same and we’re exercising more, but obesity is more prevalent than ever, does that mean we're burning fewer calories as a result of a reduced metabolic rate? And if so, is it linoleic acid's fault?
There’s evidence going both ways.
We're not gaining weight because we're suddenly eating many more calories, and we're not gaining weight because everyone started spontaneously exercising less. It is possible that our metabolic rate as a population is decreasing, but the evidence is mixed.
In the case of vegetable oils, there is evidence that more linoleic acid increases calorie burning [*,*,*,*,*], but there is also evidence suggesting it doesn't [*,*,*,*]. There are likely other mechanisms or variables at play outside of exercise or calories alone.
The Obesogenic Toxin 4-Hydroxynonenal (HNE)
One mechanism that may account for the link between increased vegetable oil consumption and obesity, is what happens when vegetable oil’s primary fat, linoleic acid, breaks down in the body.
Like a hand grenade blowing up into destructive shrapnel, linoleic acid breaks down into a number of different molecules, such as OXLAMs (oxidized linoleic acid metabolites) and aldehydes. Aldehydes are also found in cigarette smoke and include acetaldehyde, acrolein, and formaldehyde. One of the most abundant breakdown products of linoleic acid, and the most toxic of the different aldehydes, is 4-Hydroxynonenal, also called 4-HNE or HNE for short [*].
In the body, the aldehyde HNE is derived from only one source, the breakdown of omega-6 fats, primarily linoleic acid [*,*].
Toxins like HNE alter our metabolic cycle and damage fat cells, resulting in fat gain as an unfortunate side effect of excessive linoleic acid consumption, to which our body is not adapted.
Unlike other aldehydes, which are more easily managed by our antioxidant system, HNE is “highly toxic” and "tremendously reactive and long-lived,” and acts not only on the membranes in its immediate proximity, but also, "can diffuse from the site of its origin and damage even distant targets" according to scientists [*].
High levels of HNE are linked to a number of pathologies, including Alzheimer's disease, cancer, cardiovascular diseases, diabetes, liver disease, and Parkinson's disease [*]. In addition to these chronic diseases, HNE also plays a role in fat accumulation across many species, including humans [*].
In 2001, Austrian researchers at the University of Graz and University of Vienna treated single cell yeast with small amounts of HNE. While the cells untreated with HNE had little to no fat accumulation, the HNE-treated cells showed significant fat accumulation within 2 hours. After 11 days, the HNE-treated cells had either accumulated fat or had died [*].
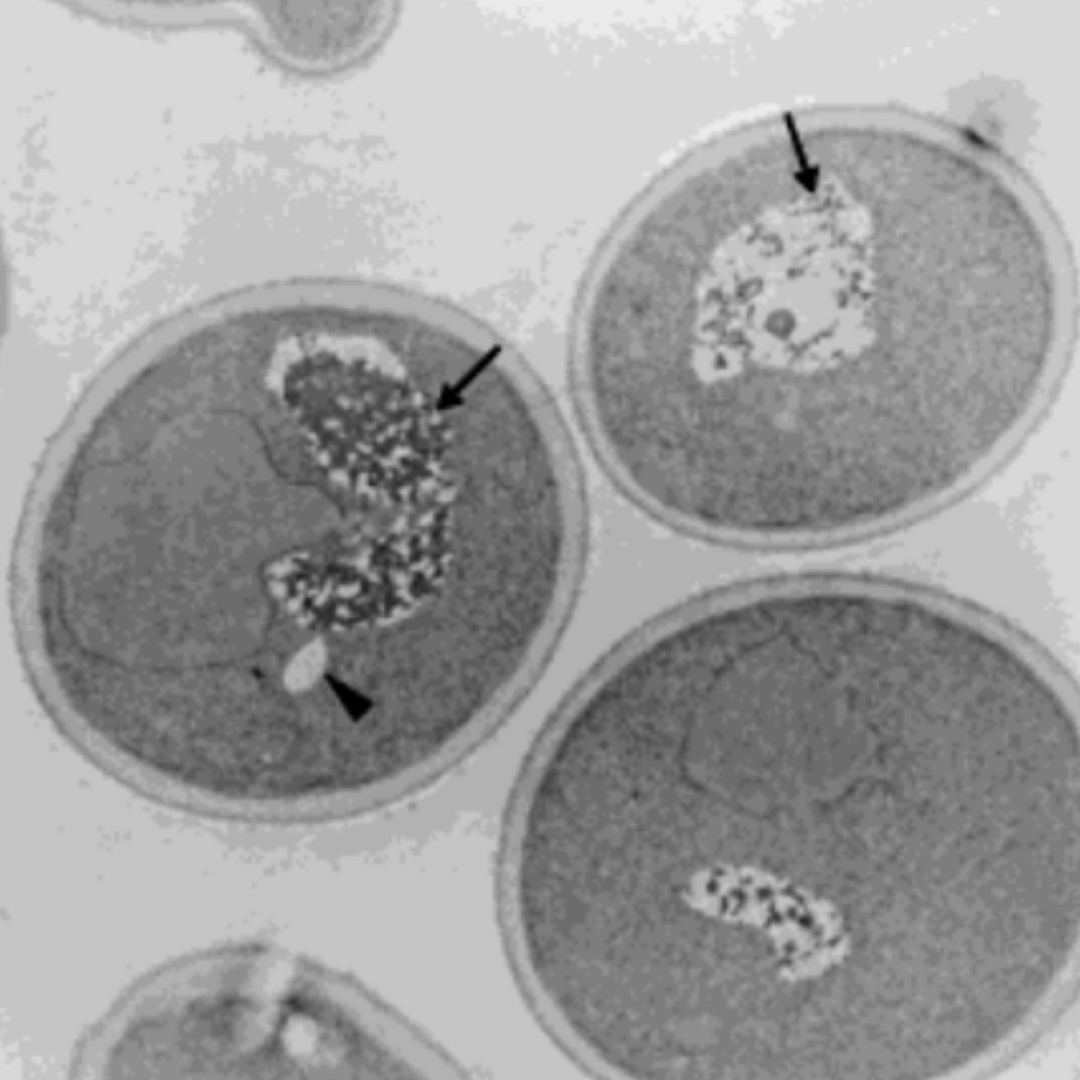
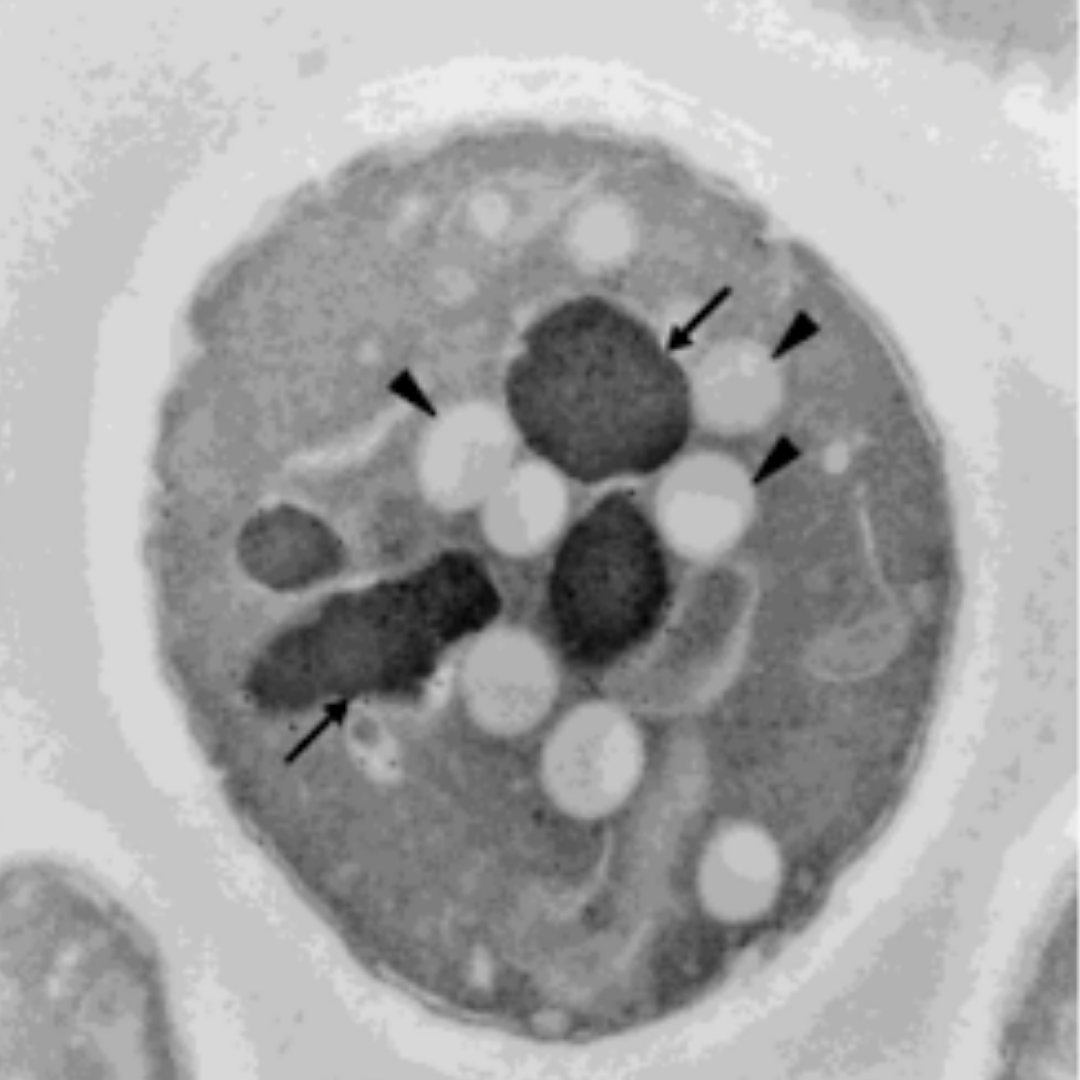
Left panel shows the control cell with no HNE treatment having intact vacuoles (arrows) and small amounts of lipids (arrow head). The right panel shows the cell treated with HNE for 2 hours having broken vacuoles (arrows) and large amounts of lipids (arrow head).
A few years later, in 2008, researchers at the University of Arkansas scaled the experiment to C. elegans, a microscopic roundworm with about a thousand cells. Similar to the single-celled yeast, the roundworms treated with HNE accumulated intracellular fat. The more HNE, the fatter the worms.

The researchers concluded that the accumulation of body fat, also known as adipose tissue, can be reversed by reducing the presence of HNE in the body, even without reducing total food intake.
In their own words, "[T]he metabolic 'pro-adipose bias' observed in the well-fed state can be reversed not only by withdrawal of food, but also by withdrawal of 4-HNE, even in the continuing presence of excess food.”
The same study showed that a reduction of HNE led to lean worms [*].
While humans are not yeast or worms, the fact that HNE leads to fat accumulation in both single-cell yeast and multi-cell roundworms suggests a common mechanism at play that may even extend to humans with elevated levels of HNE. In other words, HNE leading to fat accumulation is consistent across various species over long periods.
One of the most commonly observed genetic mutations in humans is called ALDH2*2 [*]. People with the mutation have a reduced ability to detoxify aldehydes like HNE, thereby leading to significantly increased HNE levels [*].
In December 2020, researchers in Taiwan demonstrated the causal effect of ALDH2*2 on fat accumulation in mammals for the first time. The researchers inserted the human ALDH2*2 mutation into mice and observed their food intake, activity levels, body temperatures, and weights.
The mice with the ALDH2*2 mutation had elevated levels of HNE, as expected, and while they ate the same number of calories and exhibited the same amount of movement as the mice without the mutation, they burned fewer calories and gained more weight.
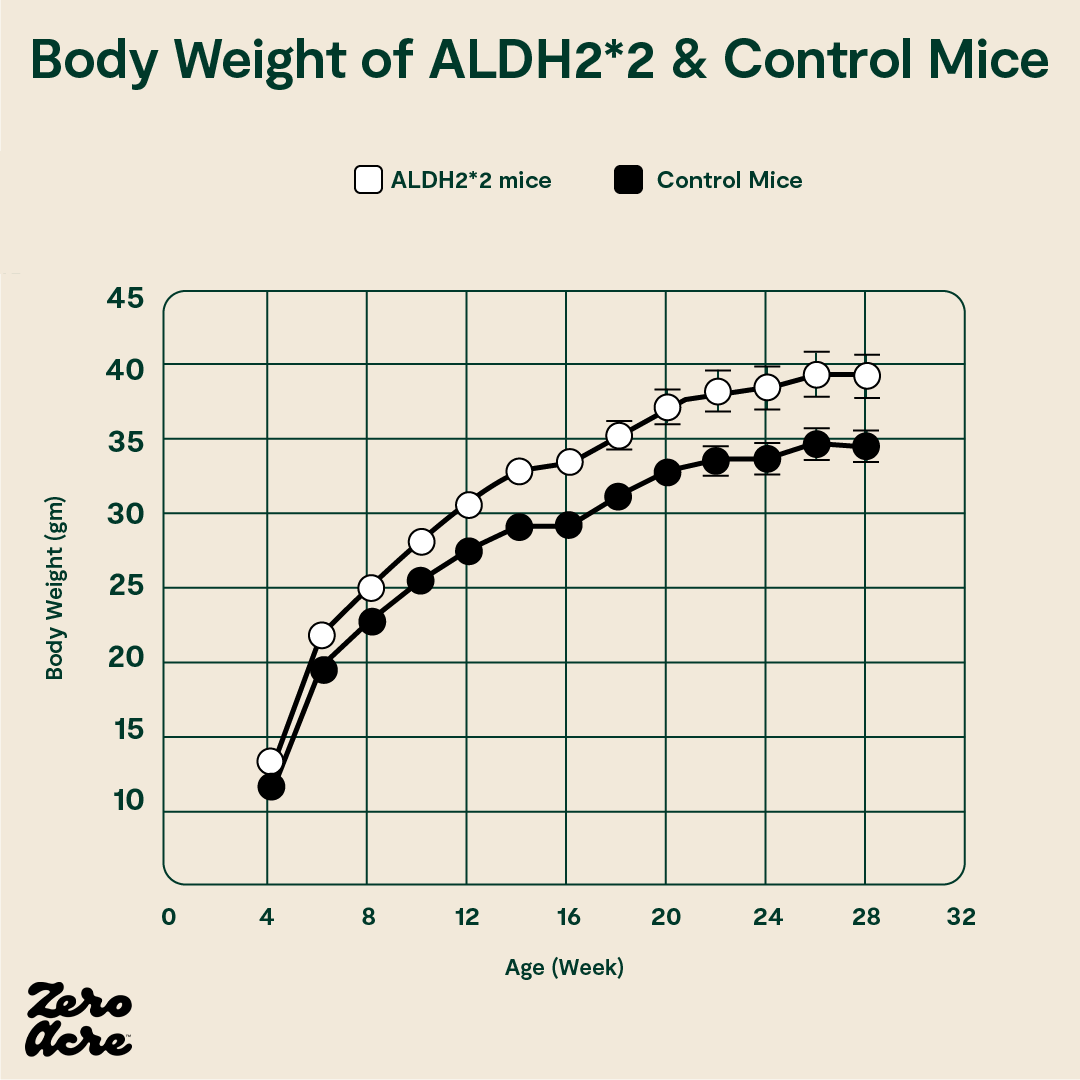
The ALDH2*2 mutant mice (white open circles) weigh more than the control mice (filled black circles).
All the mice had similar levels of physical activity, but energy expenditure and thermogenesis (the body’s ability to use calories to generate heat) were both reduced in the ALDH2*2 mice with elevated HNE levels.
In other words, the ALDH2*2 mutation, which resulted in higher levels of HNE, didn’t affect the amount of food the mice ate or how much they moved. But, it did change how many calories they burned during their lives, presumably by reducing their metabolic rate, resulting in diet-induced obesity.
When the obese mice were treated with an activator of HNE detoxification, thereby reducing HNE levels, diet-induced obesity was reversed [*]. And studies in other mammals show similar results — that higher levels of HNE are linked to increased body fat [*,*,*].
In East Asia an estimated 560 million people have the ALDH2*2 mutation. These people have significant losses of ALDH2 activity in various tissues. They also have a higher incidence of obesity [*,*,*]. And although HNE levels weren’t measured here, the reduced aldehyde detoxification efficiency resulting from the ALDH2*2 mutation most likely leads to higher levels of HNE [*]. If levels of the latter are indeed higher, they may account for the increased incidence of obesity in these people.
In a study comparing HNE levels in obese diabetics, non-obese diabetics, and endurance-trained athletes, the obese individuals had significantly elevated levels of HNE compared to lean individuals, and the endurance-trained athletes had the lowest levels of HNE [*].

A comparison of muscle HNE levels between lean, obese, and endurance-trained subjects. The 300% increase in 4-HNE in the obese subjects compared to the lean ones was statistically significant (**). The 45% decrease in 4-HNE in the endurance trained subjects compared to the lean ones was statistically significant, as was the decrease between the endurance trained subjects and the obese ones (†).
Additional research has demonstrated that exercise reduces HNE, implying that exercise may not cause fat loss simply because of the extra calories burned but because it lowers HNE levels [*,*].
In another study in humans, two different diets were tested to determine their effect on various health markers, including HNE levels. Compared to the group eating the American Heart Association-approved high-carb breakfast of a bagel, cheese, fruit, and milk, the group eating the high-fat, fast-food diet of a burger, fries, and drink had significantly elevated levels of HNE in their blood within minutes to hours [*].
Since HNE is only derived from omega-6 fats, especially from linoleic acid, it is unsurprising that the high-carb low-fat AHA-approved meal, nearly devoid of linoleic acid, resulted in lower levels of HNE compared to the fast food meal containing french fries, which are high in linoleic acid from vegetable oils.
Separate research has sought to determine the levels of HNE in different fast food items, including french fries, typically cooked in high-linoleic oils. In a 2015 study featured by the American Oil Chemists’ Society (AOCS), University of Minnesota researchers sum up their findings:
“French fries which contained higher levels of linoleic acid also contained more HNE. It is clear that HNE is produced during the heating process of the frying oils and is incorporated into french fries. Frequently consumed foods containing considerable amounts of HNE, a toxic aldehyde, may be a public health concern since HNE toxicity is related to a number of common pathological conditions.” [*]
On a study of fried chicken from fast food restaurants, researchers also found high amounts of linoleic acid and HNE in samples of chicken breast, chicken thigh, and chicken nuggets cooked in vegetable oil [*].
While HNE is produced in our bodies from the breakdown of omega-6 fats, it is also produced in frying oils subjected to heating due to the breakdown of omega-6 fats even before they enter our bodies. Consequently, fried foods cooked in repeatedly heated high-linoleic oils appear particularly obesogenic.
A study of 33,542 Europeans found that those in the highest quintile (highest 20%) of fried food consumption eat 26% of calories from fried food and 2,708 total daily calories. In contrast, those in the lowest quintile eat only 6% of calories from fried food but consume 2,738 total calories per day. Although the highest quintile of fast-food eaters consume fewer total calories, they are 26% more likely to be obese compared to those in the lowest quintile, who eat far less fried food [*].
In a randomized trial on rabbits, three groups of rabbits were given access to identical foods, with only one difference: the first group of rabbits was fed unheated vegetable oil, the second group was fed vegetable oil that had been heated once, and the third group was fed vegetable oil that had been repeatedly heated multiple times. Everything else about their diets was kept the same [*].
Compared to the group of rabbits eating unheated oil, the group eating single-heated oil gained 6% more weight, and the group eating repeatedly heated oil gained 45% more weight.
The group eating repeatedly heated vegetable oil — analogous to fried food oils — was actually consuming slightly fewer calories than the other groups and still managed to gain significantly more weight.
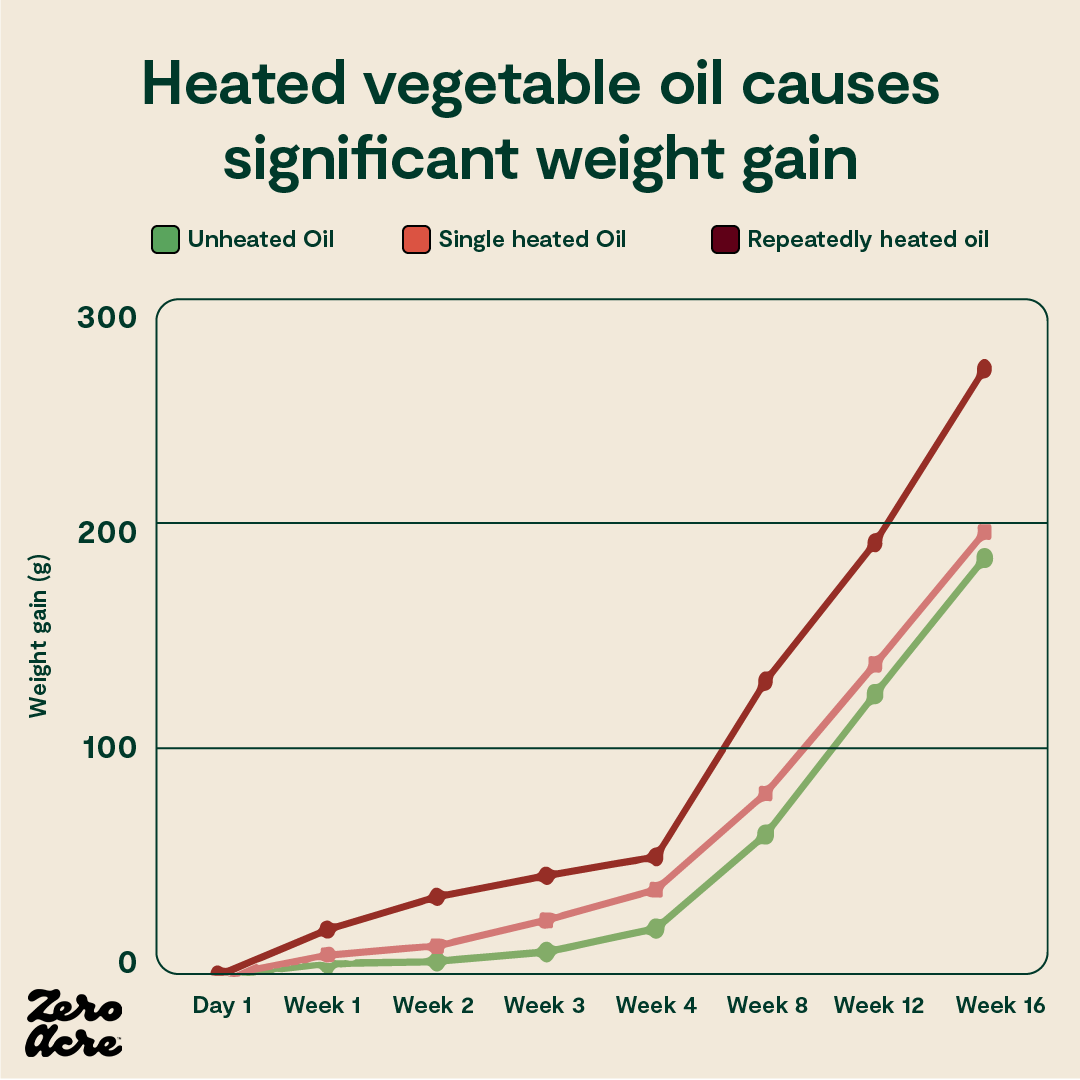
A line graph showing that the more vegetable oils are heated, the more rapid and consequent the weight gain is for rabbits consuming these oils.
There is a consensus among nutrition researchers that fried foods are linked to obesity (and disease). Still, it doesn't appear to be the caloric density of fried foods that makes it so, but rather, possibly, the content of linoleic acid-derived HNE [*,*].
It appears that HNE disrupts the process by which cells determine what to do with calories and how much fat to store across several species.
The researchers from the University of Arkansas who conducted the previously mentioned HNE studies on yeast and mice conclude, “elevated HNE realigns metabolism to favor fat deposition [...] We propose that these two partial processes — fat-induced HNE generation and HNE-triggered fat accumulation — form a positive feedback loop that perpetuates lipid storage as long as food is available [...] the link between abundance of HNE and fat storage is conserved between evolutionarily distant species” [*].
Numerous studies and lines of evidence point to linoleic acid-derived HNE as a causal route in diet-induced obesity. There are multiple strategies to decrease HNE levels, the most effective of which may be the avoidance of foods cooked in high-linoleic vegetable oils (to reduce the intake of HNE, which is derived only from omega-6 fats), in addition to exercise (to increase the detoxification of HNE). But there may be another way to reduce the burden of HNE — by restricting carbohydrates, which will be discussed later [*].
We’ve explored the fact that obesity rates across the globe have skyrocketed since the widespread increase in dietary linoleic acid. And we’ve investigated the studies behind a metabolite of linoleic acid, the obesogenic toxin HNE, that may explain the connection. Now we’ll break down how linoleic acid itself affects fat cells and our sensitivity to the fat storing hormone called insulin.
Linoleic Acid and Increased Fat Storage
Another way linoleic acid can be obesogenic is by modulating how much energy fat cells take up. These cells are exquisitely sensitive to the hormone insulin [*]. Insulin release is primarily stimulated by eating and mostly by carbohydrates.
Normal increases in insulin shut off lipolysis, the process whereby a fat cell breaks down and releases fat into the bloodstream. In other words, when you eat carbs, your body stops breaking down stored body fat for energy. When you stop eating, your insulin levels should drop far enough to turn lipolysis back on.
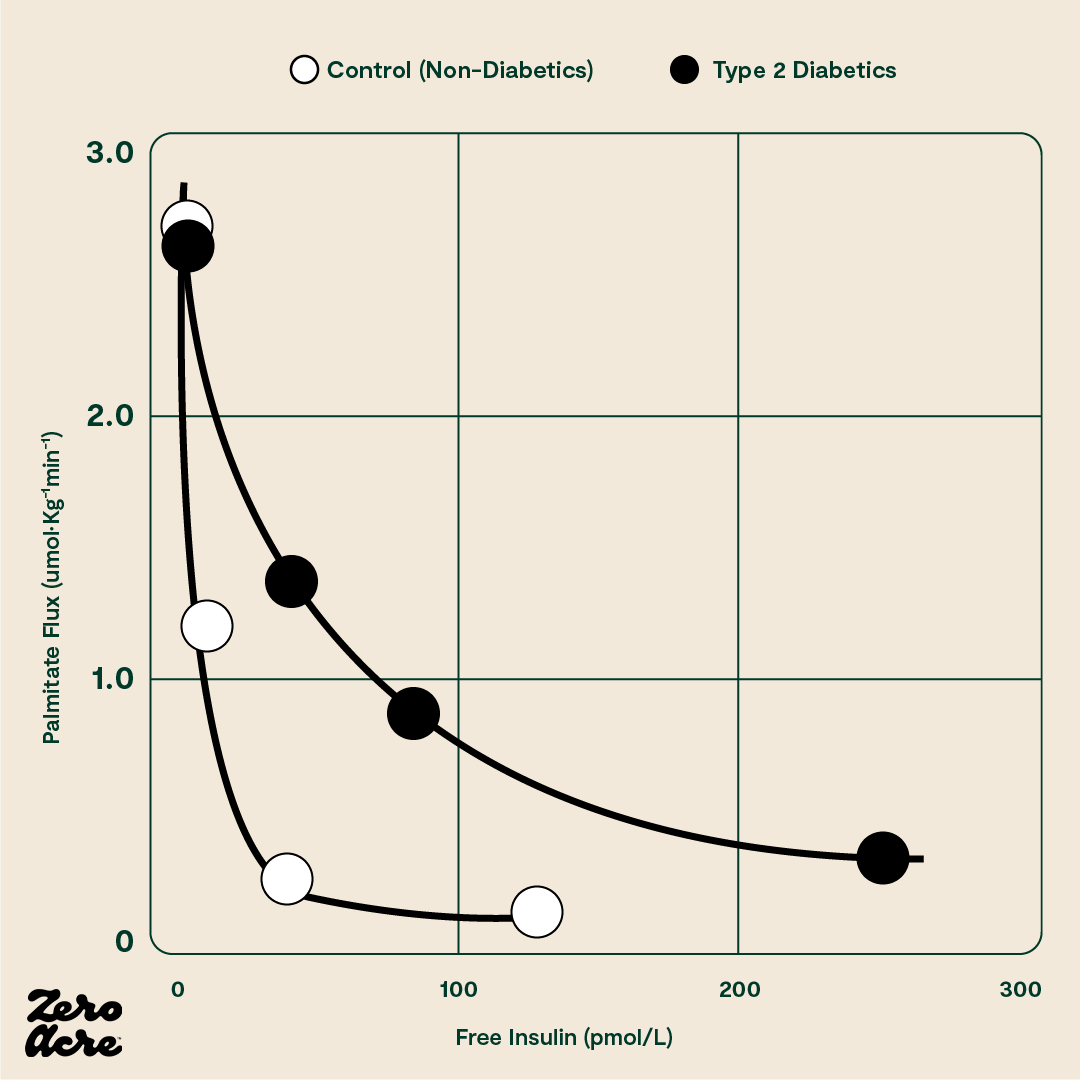
The sharp drop in the curve indicates that only a small amount of insulin is necessary to shut off the fat cell’s release of fat in a healthy person. The vertical axis plots the amount of the fatty acid palmitate moving out of the fat cell in picomoles (pM). The horizontal axis plots the amount of free (i.e. unbound) insulin in the blood in picomoles. The filled black circles (●) represent type 2 diabetics and the filled white circles (O) represent 'control' subjects who don't have type 2 diabetes.
Linoleic acid’s chemistry gives it two double bonds. Because of that, it can increase a fat cell’s sensitivity to insulin through something called redox biology [*,*,*], especially compared to fats with fewer double bonds like oleic acid (with one) or stearic acid (with none). The more insulin sensitive a fat cell is, the idea goes, the higher its propensity to store fat instead of releasing it. So, regardless of how many calories you eat, the more sensitive your fat cells are to insulin, the more likely that energy will be stored instead of released. How might that happen?
Back to redox biology: when linoleic acid is taken up by fat cells and transported into their mitochondria (the power center of the cell) for generating cellular energy, also called ATP, this process gives off a certain amount of reactive oxygen species (ROS). ROS are fundamental signaling molecules. One way fat cells use ROS is to figure out how much energy to allow into the cell [*]. When ROS reach certain levels, the fat cell may then naturally become insulin resistant; i.e., insulin receptors are removed from the cell’s surface, thus limiting further energy from entering.


It’s not just fat cells that use this mechanism, brain cells do too [*]. Think of it as the cell’s negative feedback loop system protecting it from energy overload. It is proposed that excessive dietary linoleic acid may break that loop, and the resulting failure to generate sufficient ROS would not induce insulin resistance.
Linoleic acid may not produce sufficient ROS because it contains two double bonds, and the more the double bonds fats have, the less ROS they produce [*]. There’s more complexity to this ROS-double bond relationship, but that’s the main idea. Saturated fats have no double bonds, so they produce more ROS relative to other fats that have one or more.
There’s an additional way to affect the insulin sensitivity of fat cells via cannabinoid receptor 1 (CB1). These receptors are part of our endocannabinoid system that populates many different tissues in our body. Think of this system as a dial that can rev up or tone down other systems in our bodies. As CB1 also affects the insulin sensitivity of fat cells, researchers created mice without the CB1 receptor on their fat cells. They then put them and control mice on standard chow or the infamous “high-fat diet” rich in linoleic acid [*]. The mice without CB1 receptors on their fat cells eating the high-fat diet gained the most fat, and the normal controls eating standard chow gained the least.
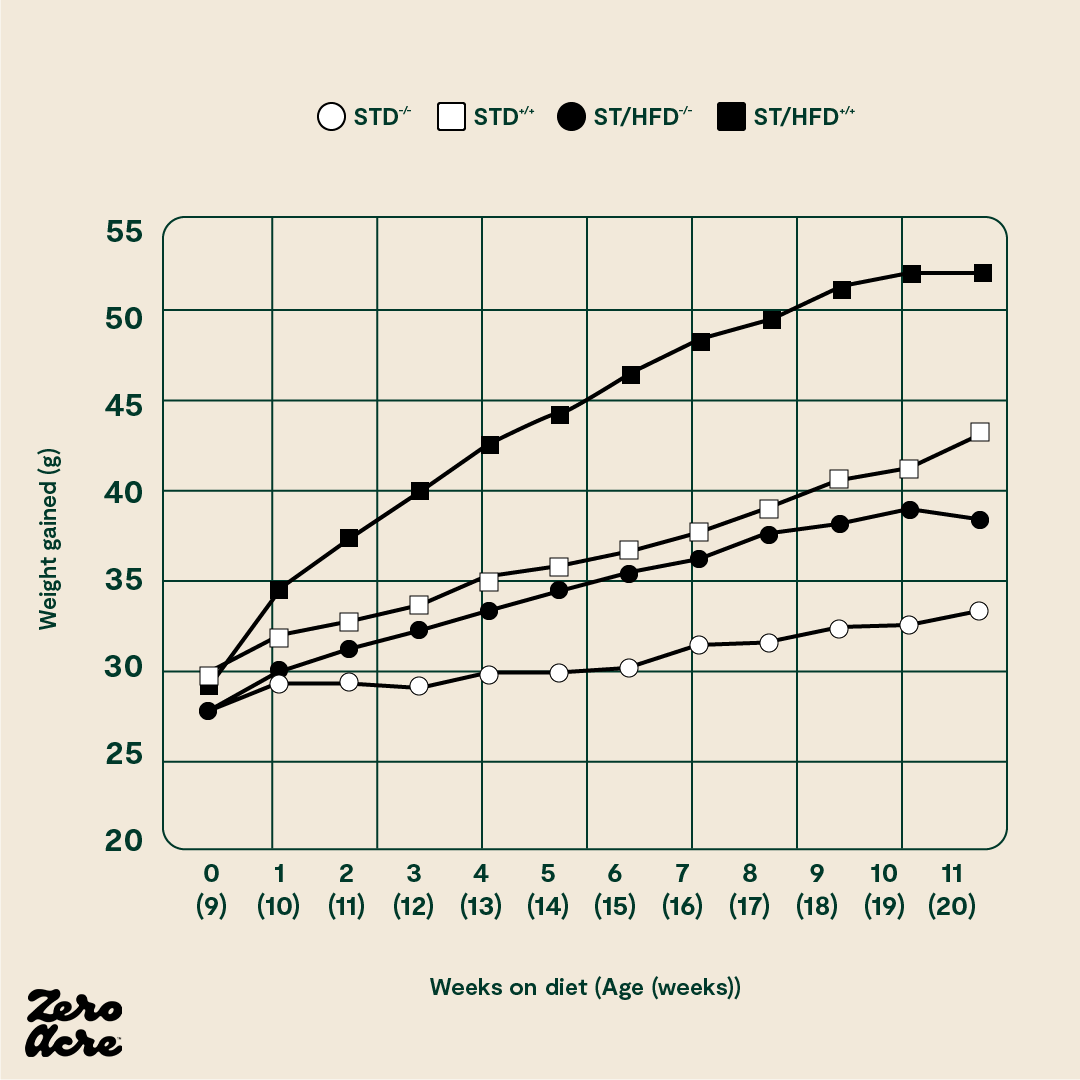
The STD -/- mice have no cannabinoid 1 (CB1) receptors and are fed a standard low-fat chow 'control' diet. The STD +/+ mice have CB1 receptors and are fed the control diet. The ST/HFD -/- mice have no CB1 receptors and are fed an obesogenic 45% fat diet. The ST/HFD +/+ mice have CB1 receptors and are fed an obesogenic 45% fat diet. The horizontal axis shows the number of weeks the mice are eating their diet as well as their age, also in weeks.
It's worth noting that the mice without CB1 receptors eating the linoleic acid-rich, high-fat diet still got somewhat fatter. This indicates that the CB1 receptor may only partially explain how humans and mice become obese.
Are Vegetable Oils Giving Us Chronic Munchies?
In addition to affecting the insulin sensitivity of fat cells, there’s yet another way that high-linoleic vegetable oils may contribute to fat gain.
We’ve all heard of “the munchies,” the acute effect that THC — the psychoactive compound in cannabis — has on appetite. Even on a full stomach, hunger cravings kick in, and all of a sudden, the pizza box and bag of chips are empty. The effects of THC are so well-recognized that it is an FDA-approved prescription drug to stimulate appetite [*].
Only in the last few years have researchers begun to understand how the same mechanisms that give us the munchies (and help sick patients to eat) may also be involved in obesity.
It turns out that we, and all animals with a spinal cord, have receptors in our brains dubbed "CB1," and those receptors are activated by THC like a key fitting into a lock. And the activation of CB1 receptors stimulates appetite, leading to increased levels of hunger.
But THC isn't the only compound that can activate CB1 receptors. While THC is a cannabinoid, we also have what are called "endocannabinoids" inside our bodies ("endo" meaning internal or within). The two primary endocannabinoids are "2-AG" and “AEA."
These endocannabinoids, 2-AG and AEA, can also activate CB1, causing hunger, and are made in our bodies from one source and one source only: arachidonic acid [*]. Arachidonic acid is found in extremely small amounts in the diet, but our bodies can also make arachidonic acid from its precursor, linoleic acid [*].
In other words, when we consume linoleic acid from vegetable oils, some of it is converted to arachidonic acid, which is then converted to the endocannabinoids 2-AG and AEA, which stimulate our appetite, and may cause us to eat more. Obese individuals tend to have much higher levels of 2-AG and AEA circling their bloodstream compared to lean individuals [*,*,*,*,*,*].
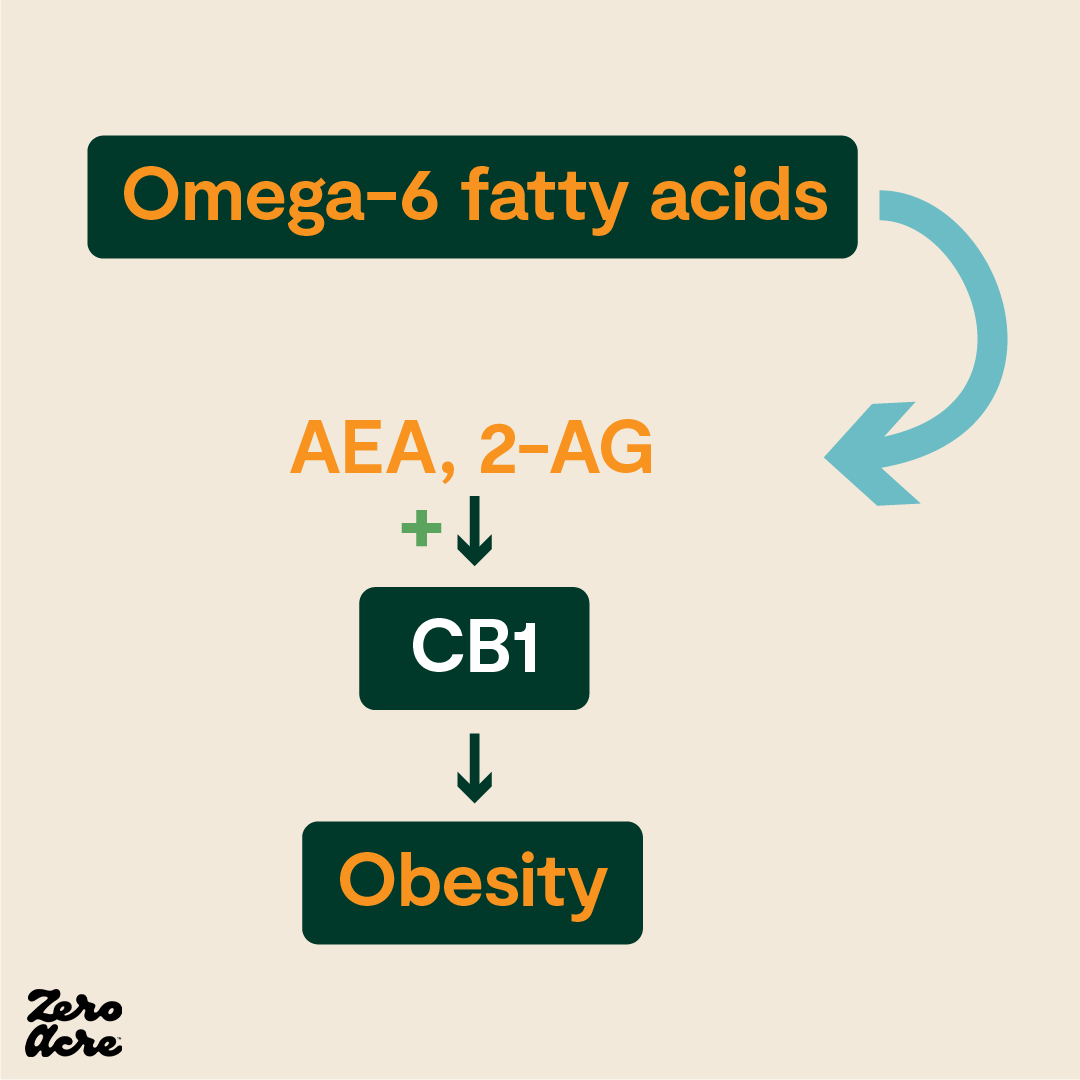
“The modern western diet, with its elevated omega-6/omega-3 ratio, leads to excess production of AEA and 2-AG. This overstimulates CB1, leading to fat gain and metabolic dysregulation" [*].
Since the endocannabinoid system is conserved across all mammals, the results of rodent studies are improving our understanding of linoleic acid's impact on 2-AG, AEA, and obesity.
In 2012, researchers at the National Institutes of Health (NIH) split mice into different groups receiving different types of high-fat chow. In the first group of mice, the chow contained 8% of calories as linoleic acid, while in the second group, the chow contained 1% of calories as linoleic acid. Everything else in the diets of the two groups remained the same; the only difference between the groups was the types of dietary fat included in the chow. All mice had 24/7 unrestricted access to their food, which is the case for most modern humans in developed countries.
The NIH researchers found that the mice eating 8% of their calories as linoleic acid gained significantly more weight than mice eating 1% of their calories as linoleic acid [*]. The extra weight gain in the mice eating more linoleic acid followed the pattern we would expect: the mice on the higher linoleic acid diet had elevated levels of 2-AG and AEA, consumed 18% more calories, and weighed 20% more than the mice eating a low linoleic acid diet.
The researchers intentionally chose 1% versus 8% of calories as linoleic acid to model the 20th-century increases of linoleic acid in Western countries, which, as the authors point out, closely correlate with increasing rates of obesity.
The researchers went on to replicate the results in more mice and even in salmon [*,*]. When salmon are fed high linoleic diets and then fed to mice, the mice gain more weight than if they had eaten salmon fed low linoleic diets.
Numerous other studies have demonstrated the obesogenic effects of linoleic acid from vegetable oils [*,*,*,*,*], but this study was the first to isolate linoleic acid as a dietary variable and show that increased levels of dietary linoleic acid directly result in increased levels of 2-AG and AEA in mammals, resulting in weight gain.
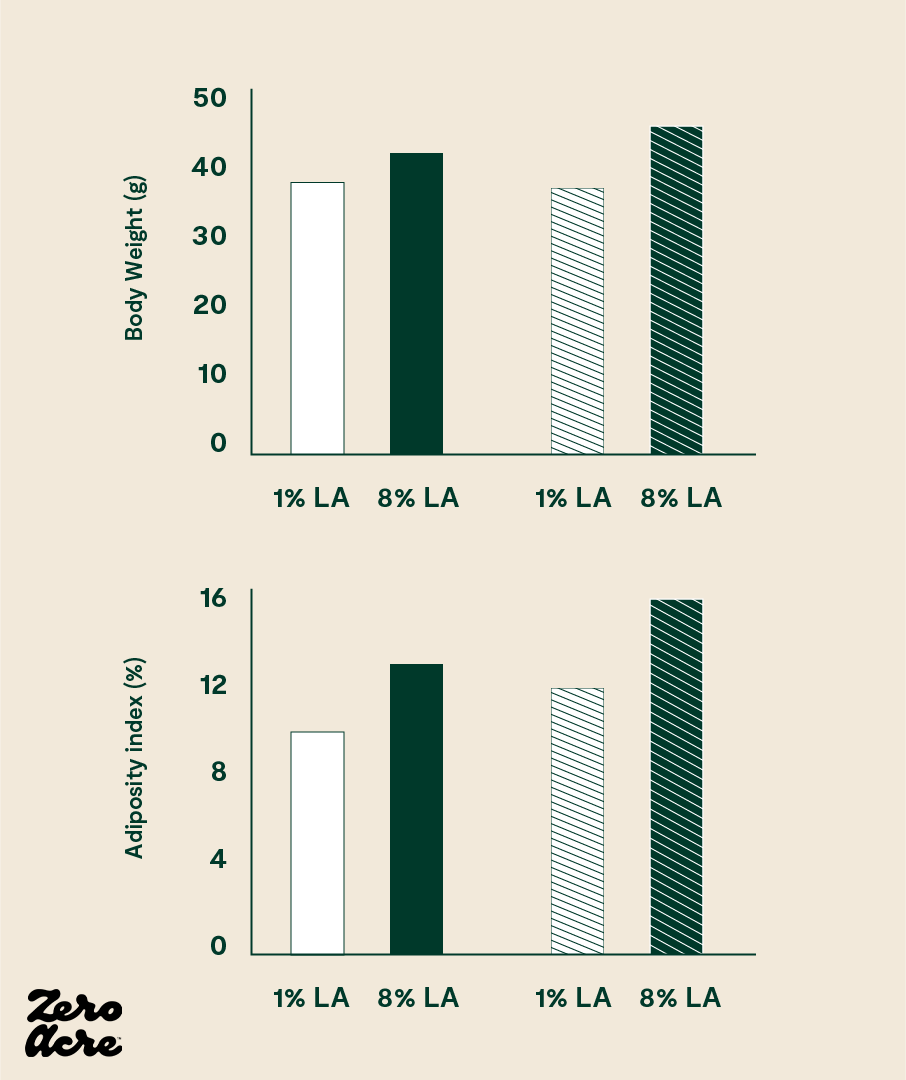
Linoleic acid dose-dependently increases body weight, body fat percentage, feed efficiency and food intake in mice (and likely in humans as well). The striped bars represent mice fed high-fat diets and the open bars refer to mice fed medium-fat diets.
In a human study, people who consumed high linoleic acid soybean oil (SO) as part of a meal consumed more calories and had lower self-reported feelings of fullness compared to people who consumed the same meal, but with lower linoleic acid oils (HOSO and VOO in the chart below). The group eating less linoleic acid had increased levels of the satiety hormone OEA, derived from monounsaturated fat, which has the opposite effects of 2-AG and AEA [*,*].
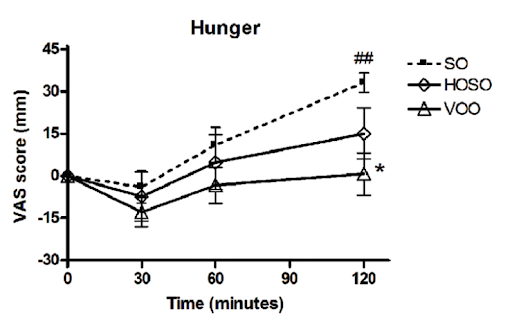
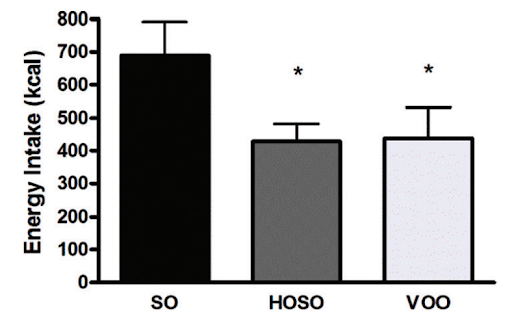
To further explore the role of the endocannabinoid system in obesity, we can also look at one of the few pharmaceutical drugs to effectively prevent and reverse obesity in humans: Rimonabant.
Rimonabant was introduced in the early 2000s as a "miracle" fat-loss pill. It helped obese individuals not only to lose fat, but also to keep it off. And Rimonabant worked by blocking CB1 receptor activity.
Patients reported being less hungry. Their hunger levels and fullness signals appeared to be working properly. Those taking Rimonabant could eat whatever they wanted and didn't get obese. Unfortunately, completely blocking CB1 had unintended consequences elsewhere in the body where the receptor resides. Suicidal ideation was one of them, forcing Rimonabant off the market. Nonetheless, the drug's effectiveness via its ability to block CB1 informed our understanding of obesity.
Furthermore, mice that are genetically engineered to have their CB1 receptors blocked do not get obese, or not nearly as much, as noted in the section on insulin-sensitive fat cells.
Bariatric surgery is another effective, albeit extreme, fat-loss intervention that also blocks CB1. The most common kind is a gastric bypass, whereby food is forced to bypass the stomach. It’s typically a last resort of morbidly obese patients who can’t seem to lose fat any other way.
While the surgery is not without its risks, its success rate is staggering. One study reported that 85% of patients lose 50% of their excess body weight, and another study showed that patients could maintain a 50-60% loss of excess weight for at least 10 years after surgery.
Although we know gastric bypass surgery works and is accompanied by reduced food intake and increased energy expenditure, in 2020, researchers at the University of Washington demonstrated that gastric bypass works in part by lowering CB1 activation in the gut [*].
The researchers also found that the effects of gastric bypass surgery could be mimicked by administering Rimonabant, demonstrating that the two interventions share similar mechanisms — downregulating or blocking CB1. And directly administering the endocannabinoid AEA, which is increased as a result of increased linoleic acid consumption, reduced the fat-loss and metabolic improvements seen with gastric bypass, again supporting the role of CB1, as well as AEA, in the development of obesity.
Once consumed, linoleic acid is eventually converted to many different metabolites in the body, including HNE, and the metabolites of arachidonic acid such as 2-AG and AEA. This conversion process adds a layer to our understanding of how this fatty acid found primarily in vegetable oils can be obesogenic.
How Linoleic Acid is Converted into Obesogenic Metabolites
The intake of linoleic acid, as well as the rate at which it is converted to more harmful molecules, are both associated with obesity.
After we consume linoleic acid, it first ends up in our blood before being incorporated into our cells. Once incorporated into our cells, linoleic acid is then converted through a cascade of reactions into other molecules, some of which, like HNE, appear to cause obesity, as discussed earlier.
In addition to linoleic acid intake, obesity is also shown to be associated with the rate at which linoleic acid is converted to gamma-linolenic acid (GLA), a precursor to arachidonic acid, by delta-6 desaturase or “D6D” for short.
Arachidonic acid is important because it’s the essential omega-6 polyunsaturated fatty acid, and is converted into possibly hunger-inducing endocannabinoids, as discussed in the previous section. Its level in different tissues is important in obesity.
For example, studies investigating obesity in Mexican, Korean, and Japanese children found correlations between obesity and increased D6D activity [*,*].
Similarly, a study comparing the weights of obese versus lean children to their cellular fatty acids found that although linoleic acid in the children’s cells did not correlate with weight, metabolites of linoleic acid — like arachidonic acid — did correlate with weight, leading the authors to speculate that the obese children may have increased D6D activity [*].
If metabolites of linoleic acid are a cause of fat gain, then it makes sense that the faster our bodies create them from the linoleic acid we eat, the more fat we’ll gain.
Indeed, studies that show that lower levels of linoleic acid in our cells are associated with more weight gain may miss the point if they do not consider the importance of D6D.
As we’ve explored above, there are indirect ways for linoleic acid to cause fat gain when certain of its downstream metabolites increase in concentration. Thus, studies that make an assumption about the effects of dietary linoleic acid based solely on tissue or blood plasma levels are misguided. It’s not enough to know the blood plasma or tissue levels of linoleic acid; you also need to know how much of its downstream metabolites are building up.
In humans, one study using self-reported food intake found that "plasma [blood] levels of linoleic acid were inversely, and dietary linoleic acid was positively associated with diabetes risk" [*].
In other words, people who had low levels of linoleic acid in their blood were more likely to develop diabetes. In contrast, people who consumed more linoleic acid were more likely to develop diabetes. Understandably, the results confused researchers, given that other studies showed blood levels of linoleic acid corresponded to dietary intake of linoleic acid. The study authors suggest that different D6D activity and insulin levels may explain the confusing results.
In study participants who have lower levels of linoleic acid in their blood, there could be one of two things happening:
Those people do, in fact, consume less linoleic acid and therefore have less linoleic acid in their cells.
Those people have increased D6D activity, which means they are genetically predisposed to convert more linoleic acid to other molecules, resulting in lower levels of linoleic acid and higher levels of its downstream metabolites.
Indeed, numerous studies demonstrate that increasing dietary linoleic acid results in increased levels of metabolites downstream of arachidonic acid. Systematic reviews find that arachidonic acid levels change very little in response to different levels of linoleic acid intake [*].
It appears that excess linoleic acid that is converted to arachidonic acid is then quickly converted to these other metabolites. The half-life of linoleic acid in fat cells has been estimated to be 680 days; if this is accurate, it means that the vegetable oils you ate several years ago are still what make up much of your fat cells [*].
Where did these biological pathways mediating obesity come from? Knowing their origin might help us better exploit them. Evolution may provide some food for thought here.
Linoleic Acid as a Hibernation Signal in Animals
In the spring and early summer months, most available foods are low in linoleic acid. For herbivores, that means leaves and greens, and for omnivores, that means leaves, greens, and the small animals that eat them. However, leading up to winter, when the leaves start to fall, the diets of wild animals become higher in seeds, nuts, and the animals that eat them, which are higher in linoleic acid.
Now you may be thinking, “What do the dietary patterns of hibernating animals have to do with me?” It turns out that hibernation exists across a wide variety of animals, in different classes and families all across the animal kingdom.
Bears aren’t the only hibernators, but also bats, squirrels, insects, frogs, chipmunks, turtles, snails, lungfish, hummingbirds, earthworms, and even primates (lemurs), suggesting that it is a highly conserved evolutionary adaptation that evolved in a common early ancestor.
Hibernation evolved as a way to survive those months of the year when little food was available. During those months, pursuing food would result in more calories burned than consumed, making it a counterproductive activity from a survival standpoint.
Hibernation allows certain animals to curl up and conserve energy by significantly decreasing metabolic rate and consuming stored body fat for sustenance. Before going into hibernation, these animals “fatten up” to increase body fat stores that must sustain them for the months ahead.
For example, brown bears have a huge spike in appetite and gain an extra 30% of their body weight as winter approaches, mostly in the form of body fat. During hibernation, all of that extra body fat is consumed for energy and the metabolic rate of hibernating bears is reduced by 50-60%.

A lean bear (left) outside of the hibernation period and a fatter bear (right) soon before entering hibernation.
During the spring, leading into the summer, bears have 8-12% linoleic acid in their body fat. However, during autumn, in the weeks leading into winter, the linoleic acid in the bears’ body fat more than doubles, increasing to 25-26% as a result of increasing linoleic acid in the diet [*].
Chipmunks and certain ground squirrels are known to switch from a diet of berries, fruits, and plants in the summer to one of acorns, seeds, and other nuts in the autumn, significantly increasing the levels of linoleic acid in their diet. Certain animals like chipmunks and squirrels also “hoard” their foods once they receive signals from their body to prepare for winter.


Squirrels tend to switch from a diet of leaves, grasses and plants in the summer (left) to one of acorns, seeds, and other nuts in the autumn (right), significantly increasing the levels of linoleic acid in their diet.
Because polyunsaturated fats like linoleic acid stay liquid at much colder temperatures than monounsaturated and saturated fats, it can be beneficial for hibernating animals to have extra linoleic acid in their bloodstream during the cold months of hibernation, ensuring fluidity in organs like the heart [*].
In numerous experiments, researchers have given soon-to-hibernate animals different diets — all identical except in the type of fat — and have observed that dietary linoleic acid dictates hibernation duration and depth in many animals living in cold climates.
Bears, which maintain a higher body temperature during hibernation, have less need for highly-liquid polyunsaturated fats like linoleic acid for their hearts and organs, but just like other mammals, appear to gain weight faster on a diet with a good portion of linoleic acid, but little omega-3 [*].
Animals with low body temperatures during hibernation, on low linoleic acid diets leading up to hibernation have much shorter and shallower hibernation periods, if they enter hibernation at all [*,*,*,*,*].
For example, in a study of 12 ground squirrels separated into three groups of four, with each group receiving different amounts of dietary linoleic acid, the group of four squirrels with the least linoleic acid never entered hibernation and had the lowest body weights, while all four squirrels in the group eating the most linoleic acid entered hibernation and had the highest average body weight [*].
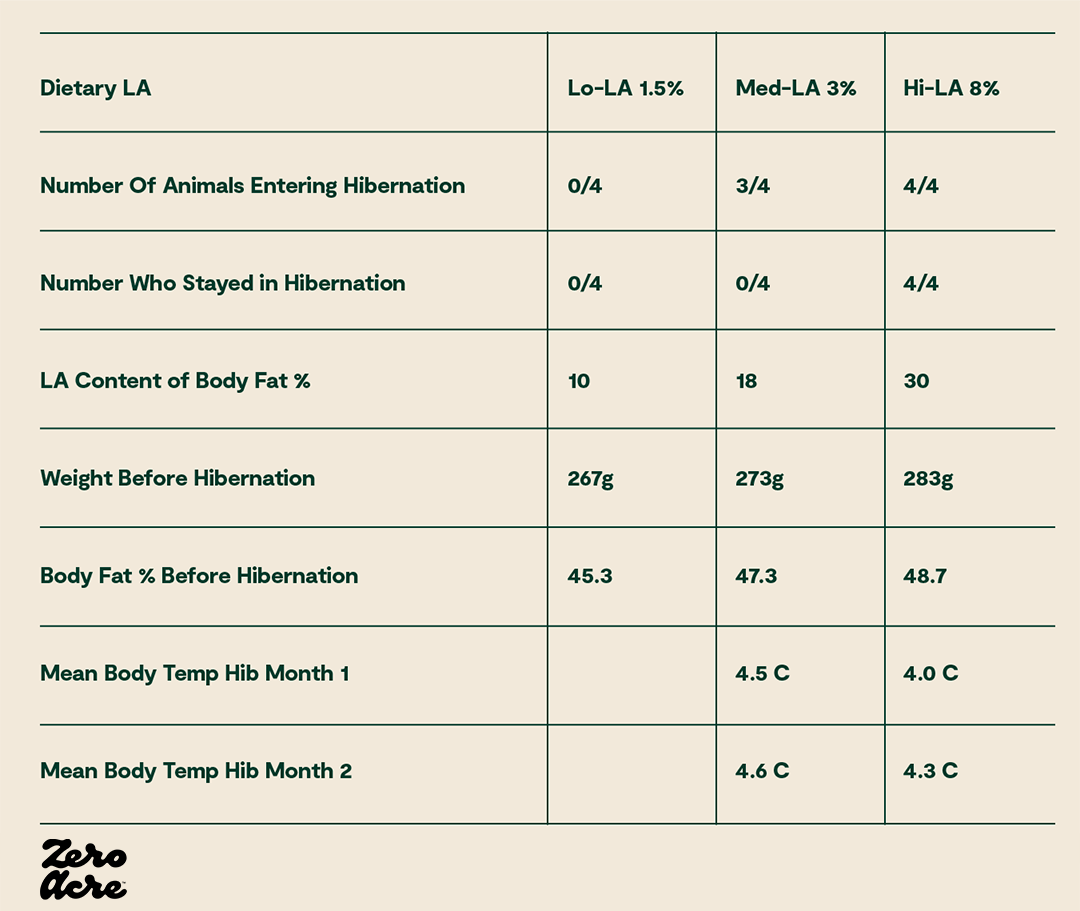
To put it another way, cold-weather hibernating mammals seem to take advantage of large amounts of linoleic acid in order to gain body fat, decrease energy expenditure, and hibernate for the winter. Conversely, without increased linoleic acid, these mammals don’t gain as much fat and have higher energy levels, which is reminiscent of the desires of most modern humans!
Interestingly, animals in warmer climates, such as that in which we evolved, avoid seed oils when they’re hibernating. Lemurs — another primate [*] — and bats [*], which both hibernate during dry periods in warm climates when food is not available, simply don’t seek out as many high-linoleic acid seeds.
Sub-tropical bats actually shift their diet to avoid unstable polyunsaturated fats and lower their body’s percentage of linoleic acid. Scientists believe that without a specific need to deal with cold temperature, the negative effects of linoleic acid during the long period of hibernation supersedes the benefits of fat gain and lower metabolism [*,*].
While humans are not adapted to hibernation [*], we share many of the same signaling pathways as animals, conserved from common ancestors millions of years ago. And those signals may push our bodies towards fat storage as a result of a high linoleic acid diet.
For example, hibernating animals have altered levels of arachidonic acid and 2-AG, the same molecules we examined in previous sections [*]. What was once an evolutionary advantage may backfire in the context of abundant food and vastly increasing amounts of dietary linoleic acid from vegetable oils.
Now that we've covered the metabolic effects of linoleic acid alone, let's look at what happens when a high linoleic acid diet is combined with carbohydrates.
Vegetable Oils and Carbohydrates
Up to this point, you may have been wondering about carbohydrates, the macronutrient behind (or rather left out of) popular weight-loss diets such as the ketogenic diet and Atkins diet.
Research shows that ketogenic diets in rats create an additional disposal pathway for HNE: it’s broken down into different forms until it can be used for fuel instead of being left to disrupt cells [*].
Well-designed ketogenic diets for humans — such as those from Virta Health, which have been clinically proven through peer-reviewed research to significantly improve health outcomes associated with obesity — not only prescribe a reduction in carbohydrates, but also a reduction in high linoleic vegetable oils [*,*].
The combination of reducing carbohydrates and high linoleic vegetable oils appears to be an especially effective weight loss combination in both human and animal studies.
In one study, mice on high-carbohydrate diets gained 11% more weight (primarily in the form of body fat) when the fat in their diet contained 8% of calories from linoleic acid versus 1%, even when total calorie consumption did not change. The study’s authors explain that diets with excess linoleic acid, when combined with carbohydrates, can cause weight gain even without causing overeating [*].
Separate research has demonstrated that activation of the CB1 receptor — a result of increased linoleic acid consumption — preferentially directs carbohydrate calories towards fat storage [*]. Instead of burning dietary carbohydrates as energy, they are stored away as fat when combined with a diet high in linoleic acid.
Unfortunately, excess linoleic acid, which results in increased levels of 2-AG and AEA and activates CB1, not only causes fat gain — even when diets are matched in calories — but also increases cravings for the combination of fat, carbohydrates, and sugar [*]. In human trials, activation of CB1 also resulted in weight gain and increased food intake, especially of snack foods such as candy bars, cookies, and cakes [*].
In other words, the combination of linoleic acid and carbohydrates may trigger a vicious cycle whereby increased linoleic acid leads to increased weight gain and cravings for snack foods, which themselves tend to be high in linoleic acid, perpetuating the cycle of overeating and weight gain [*].
Every 10% reduction in dietary carbohydrates from total energy intake increases total energy expenditure by 52 kcal/d, suggesting that carbohydrate restriction may be an effective fat-loss lever in its own right [*].
As we will see below, a high-sugar or high-starch diet with large amounts of linoleic acid is particularly obesogenic. Similarly, low-carbohydrate diets may aid in fat loss, in part because of the synergistic effects of sufficiently low levels of linoleic acid and vegetable oils.
In a January 2021 article in Nature comparing low-carb to low-fat diets, NIH nutrition researcher Kevin Hall published the results of a two-week randomized controlled trial in 20 adults that left low-carb diet advocates scratching their heads. Half of the adults in the study consumed a low-carb diet, and the other half consumed a low-fat diet. All meals and snacks were prepared by the researchers and the participants in the study could eat as many snacks as they wanted.
The group eating fewer carbohydrates consumed 34% more daily calories (2,689 vs. 2,000) and lost less body fat, but this difference in fat loss didn't reach statistical significance:

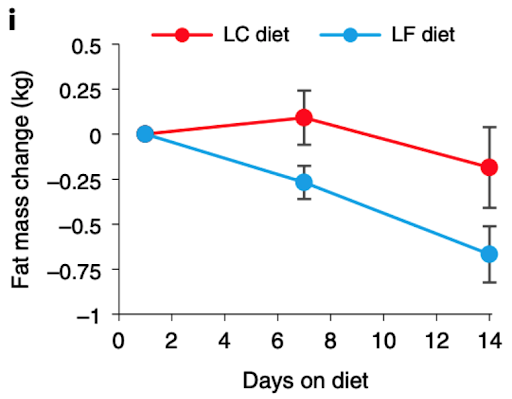
You could then ask, “Why does a low-fat diet trend towards more body fat loss than a low-carb diet in the short term?” One answer may lie in the type of fat each group was consuming. While the low-fat group was not intentionally avoiding vegetable oils, the low-fat diet ended up being a low linoleic acid diet, with only about 3% of calories coming from omega-6 (linoleic acid). However, the low-carbohydrate group had large amounts of added fats in its meals and those fats came in the form of dressings and mayonnaise made with soybean oil and other vegetable oils, resulting in 18% of calories coming from omega-6 [*].
If the low-carbohydrate group had also eaten less linoleic acid and vegetable oils, we could speculate that the results may have been different.
A study in the New England Journal of Medicine, authored by leading nutrition researchers Dariush Mozaffarian, Frank Hu, and Walter Willett, found that the consumption of carbohydrate-rich potato chips and french fries, which are simply potatoes cooked in vegetable oil, are associated with 2-6 times as much weight gain as baked or mashed potatoes, which are typically made with fats very low in linoleic acid like butter and cream [*,*]. On the other hand, potato chips and french fries are primarily cooked in high-linoleic oils such as corn, soybean, sunflower, and/or canola [*,*].
These findings give us even more insight into the role of high-linoleic acid seed oils on obesity trends, but why do some studies show that increased vegetable oil consumption does not cause weight gain?
When Vegetable Oils Don’t Cause Weight Gain
It is important to note that some studies show an increase in dietary linoleic acid and no resulting change in weight or fat. However, these studies typically have numerous flaws.
For example, we knew in 1999 that it took about four days for linoleic acid to reach its maximum conversion to arachidonic acid [*], the precursor to the appetite-increasing endocannabinoids 2-AG and AEA. Yet many studies feed participants only one meal or for less than 24 hours and unsurprisingly don’t see the same effect as studies that feed participants increased levels of linoleic acid for multiple days, weeks, or months.
Similarly, it appears that high linoleic vegetable oils are most obesogenic when heated, and especially when deep fried. So, studies looking only at the effect of unheated vegetable oil consumption may be missing the full picture — that the majority of vegetable oil consumed by modern humans is cooking oil heated to high temperatures.
Data that appear to be “conflicting” on the surface may sometimes turn out to be irrelevant when examined further.
As another example, there may be a threshold beyond which extra linoleic acid does not cause more weight gain. In animal models, there is a significant difference in weight gain between a diet that is 1% linoleic acid versus 8% linoleic acid and 4% versus 8% linoleic acid. However, there is not much difference in weight gain between an 8% linoleic acid diet and a 37% linoleic acid diet [*]. It appears that the increasingly obesogenic properties of excess linoleic acid start to level off somewhere between 5-8% of calories.
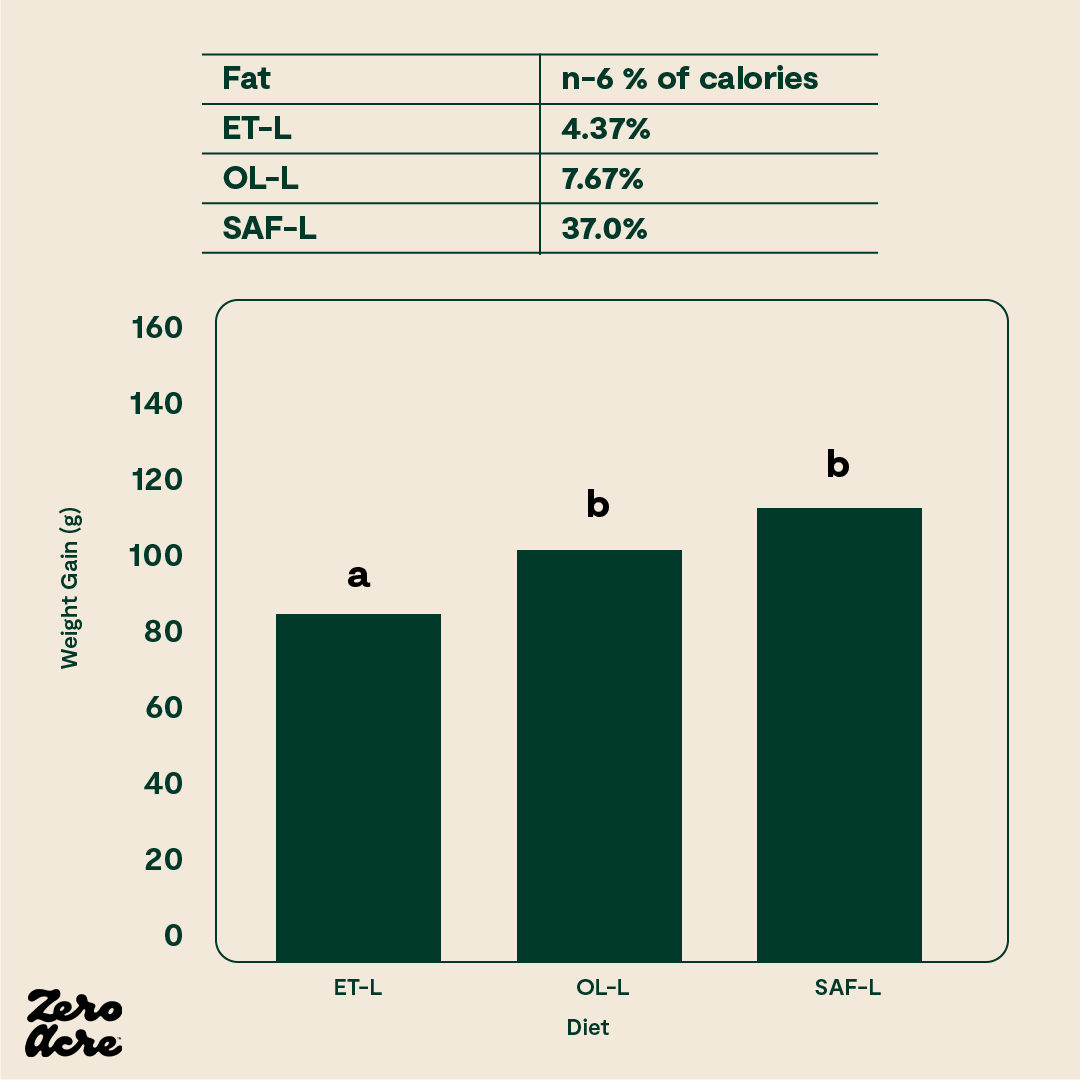
Obesogenic properties of excess linoleic acid.
In another study in mice, there was a significant difference in body weight between mice on 1% linoleic acid diets and mice on 15% linoleic acid diets, but no additional weight gain was observed for the mice eating 22.5% of calories from linoleic acid [*].
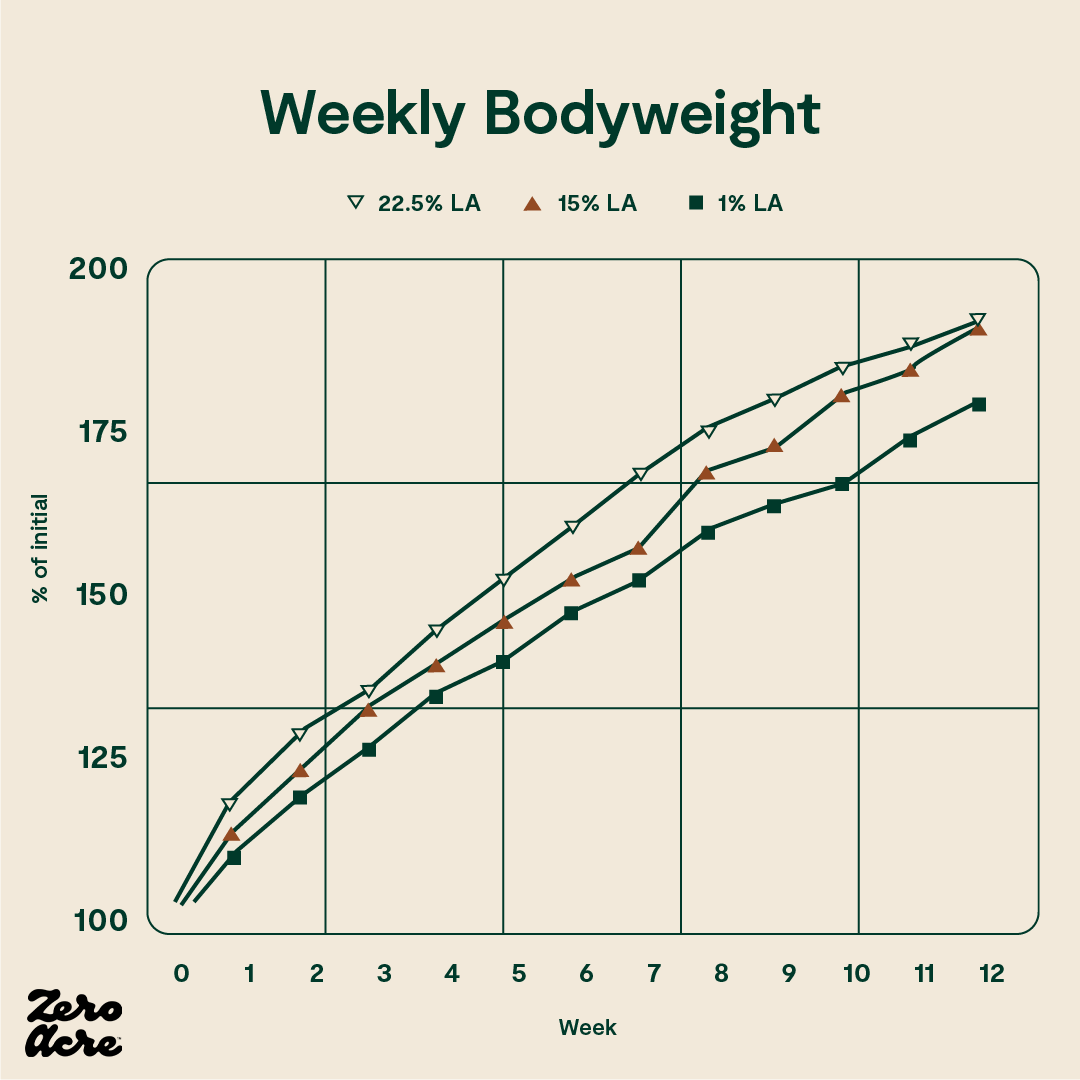
The line graph shows that the 1% linoleic acid group weighed less than the 22.5% linoleic acid group at weeks 7 and 8. From weeks 9 to 12 the 1% linoleic acid group weighed less than both the 15% and 22.5% linoleic acid groups. The STD -/- mice have no cannabinoid 1 (CB1) receptors and are fed a standard low-fat chow 'control' diet. The STD +/+ mice have CB1 receptors and are fed the control diet. The ST/HFD -/- mice are fed an obesogenic 45% fat diet. The ST/HFD +/+ mice are fed an obesogenic 45% fat diet. The horizontal axis shows the number of weeks the mice are eating their diet as well as their age, also in weeks.
However, it should be noted that while extremely high linoleic acid diets don’t seem to cause extra weight gain, other markers of poor health, such as insulin tolerance, are significantly increased on diets with large amounts of linoleic acid. It appears that after a certain point, linoleic acid in our bodies stops causing additional weight gain and starts causing other problems, leading to diabetes.

At 22% of calories from linoleic acid, this eventually overwhelms obesity’s protective action that guards us against diabetes: pathological insulin resistance and elevated blood glucose area under the curve (AUC) appear.
If extra linoleic acid past 5-8% of calories does not further contribute to weight gain, this would mean that increasing obesity rates across the world have more to do with an increasing percentage of people that reach 5-8% of their calories as linoleic acid, as opposed to an increasing amount of linoleic acid consumed overall. This may also be why the association between linoleic acid consumption and weight gain gets fuzzy past 5-8% of calories. In the context of weight gain, it may not matter much once that threshold is crossed.
In the U.S. in the year 2000, the average dietary intake of linoleic acid was estimated to be 5.5-6% of calories [*]. In 2014, that number crept up to 7.7% of calories [*].
Unless a randomized trial is very carefully controlled, a slight increase or decrease in dietary linoleic acid may not result in a weight gain difference with the backdrop of an average American diet that may already be well over 5-8% of calories as linoleic acid.
Regardless of the reasons why, conflicting evidence is inevitable in human nutrition. Just look at trans fats. It took until 2018 for the FDA to finally ban trans fats, even though there was overwhelming evidence of their harm since at least the 1990s [*].
We now know that for decades, big food companies like the makers of margarine and other industry organizations that made money from trans fats paid for studies to show that trans fats were harmless, resulting in “conflicting evidence” [*]. One industry insider even recalls being tasked with following trans fats researchers at conferences and making a point of objecting to every one of their conclusions in the Q&A following their lectures [*,*].
Only a few years ago, it was revealed that Coca-Cola did the same thing, paying for studies to show that sugar was harmless and that exercise is more important for health than sugar reduction [*].
If we can learn anything from the controversial history of trans fats and sugar, it’s that so-called conflicting evidence regarding a food’s impact on health is not an indication of insignificant harm from that food. Even foods that have conflicting evidence of harm when consumed in excess, as a result of both industry funding and the unavoidable limitations of nutrition studies, can still be leading drivers of poor health, and vegetable oils high in linoleic acid may be in that same category.
Conclusion
It becomes abundantly clear to anyone who has spent time reading through nutritional scientific literature that there’s a lot more we don’t know about the body than what we do know. We don’t yet fully understand what’s happening when different species in different environments eat different foods, or why those things happen.
With enough time and better science, we’re sure to figure it out. But until then, we have a chronic disease and obesity pandemic on our hands, and there are clear mechanistic data, animal studies, and human studies showing the obesogenic properties of excess vegetable oil consumption.
Vegetable oils are the major category of food in our diet that has increased the most in the last hundred years, during which time obesity has gone from obscurity to commonplace, so in our fight against obesity, reducing vegetable oils and their primary fat linoleic acid in our diets seems like a good place to start.
** Source: Personal communication with researcher Kevin Hall
Thank you to Tucker Goodrich, Catherine Shanahan, MD, Brad Marshall, Chris Knobbe MD, Dr. Andrew Jenkinson, Dr. Shaan Naughton, and Raphael Sirtoli, Ph.D.(c), for their insights, observations, and analyses on the topic of vegetable oils, linoleic acid, and obesity.
To continue learning:
Read Dr. Andrew Jenkinson’s book Why We Eat (Too Much)
Read Tucker Goodrich’s excellent presentation on vegetable oils and obesity
Watch Dr. Chris Knobbe’s presentation The Omega-6 Apocalypse
Read Dr. Catherine Shanahan’s book The Fatburn Fix
Read Brad Marshall’s insightful posts on linoleic acid and hibernation
Read Dr. James DiNicolantonio's book The Obesity Fix
Read Dr. Shaan Naughton comprehensive paper “Linoleic acid and the pathogenesis of obesity”
Read more about Raphael Sirtoli’s work at the Pêro Medical Clinic in Lisbon, Portugal





